MgO Functionalized Magnetic Activated Carbon Material for Efficient Removal of Malachite Green from Aqueous Solutions
Abstract
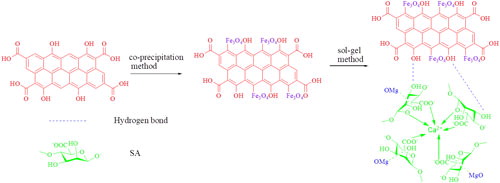
Malachite green (MG) pollution has a negative impact on human health. At present, the method of removing it is inconvenient to operate and the cost is high, which has aroused widespread concern. In this study, MgO functionalized magnetic activated carbon (MgO-mAC) prepared by the sol–gel method was used to remove MG in water. The physical and chemical properties of MgO-mAC were tested by SEM, TEM, FTIR, XRD, BET and VSM. The effects of adsorbent dosage, solution pH, contact time, initial MG concentration and temperature on adsorption were studied by batch experiments. The adsorption kinetics data is well described by a pseudo-second-order model. The equilibrium data fits the Langmuir isotherm well. When the pH is 8 and the contact time is 360min, the maximum adsorption capacity of MG is 3809mg⋅g−1. In thermodynamic studies, ΔG∘<0, ΔH∘>0, ΔS>0, MG adsorption is an endothermic and spontaneous adsorption process. The current synthesis method is simple in operation and cheap in raw materials, which can greatly reduce the cost of synthesis. Hence, the MgO-mAC material will be an effective adsorbent for removing MG from aqueous solutions.