Photoacoustic effect invokes auditory response in infrared neuron stimulation
Abstract
Infrared neuron stimulation is regarded as an innovative approach for stimulating cochleae in animals while the exact mechanism still remains unknown. In this paper, we studied compound action potentials of guinea pig cochleae with chronic or acute deafness. We recorded optical compound action potentials and analyzed stretched cochlear preparations by fluorescence microscopy. Photoacoustic signals were measured by hydrophone and microphone, respectively. In our experiment, we observed a switch response effect in vitro and in vivo experiments. Therefore, we proposed photoacoustic effect could invoke auditory response in infrared neuron stimulation.
1. Introduction
Since Dr. William House first inserted a gold wire into the cochleae of deaf patients in 1961,1,2 the cochlear implant (CI) has been widely regarded as the most effective method to restore hearing in patients with severe sensorineural hearing loss. Still, the electrical stimulation of CI has some inevitable deficiencies, including low spatial resolution and artifacts.3 Infrared neuron stimulation (INS) has recently been proposed as an alternative to overcome these issues and improve CI performance. Previously, there have been several groups researching in INS. Izzo and Richter, etc. in Northwestern University first proposed a mid-infrared pulsed laser to successfully evoke OCAPs in the gerbils.4 They proceeded to studying the effects to OCAPs with different stimulation parameters including stimulation position,5 stimulation rate,6,7 stimulation pulse duration8,9 and so on. Wenzel and Teudt, another research group in Medical University Hannover, applied a green laser with nanosecond pulse duration to induce the auditory path after destroying the round window in the cochlear and acquired optically evoked auditory brainstem response (OABR). Jonathon Wells in Vanderbilt University measured the responses of compound nerve action potentials through a pulsed low-energy infrared laser stimulation in a rat sciatic nerve model. He discussed the possible mechanisms of INS and suggested the neural activation was induced by a thermal transient.10 Matthew Keller showed availability of a three channel, wearable laser pack, whose laser source had been miniaturized several orders of magnitude to facilitate chronic animal studies.
Despite ongoing research, the exact mechanism underlying infrared neuron stimulation of the auditory nerve remains unknown. Some reseachers regarded photothermal as a potential mechanism for infrared neuron stimulation of the auditory nerve. In 2006, Izzo and colleagues first applied 2120nm laser with pulse duration of 250μs to test acute and chronic deafness gerbils whose cochleae lacked outer and inner hair cells (HCs). They proposed the interactions between light and tissue may be photochemical, photomechanical, or photothermal in nature.11 Using a pulsed diode laser (1940nm), they stimulated auditory neurons of deaf gerbils at pulse durations of 5, 10, 30, 100 and 300ns and determined that water absorption of optical radiation was a significant factor in stimulation. They suggested that the mechanism was likely a small, transient increase in tissue temperature upon light absorption by water.12,13 In animals with acute or chronic deafness induced by neomycin application into the middle ear, Richter and colleagues applied 1844–1873nm laser with a pulse duration of 30–1600μs to explore the correlation between optical stimulation and spiral ganglion neuron (SGN) survival. The stimulated section along the cochlear spiral ganglion was estimated from neural responses recorded from the central nucleus of the inferior colliculus. Stimulation resulted in a spatially and temporally confined increase in tissue temperature upon light absorption.14,15 Rather than gerbils, Rajguru et al. used cats and an 1850nm pulsed laser with a pulse duration of 100μs and observed neural response. They proposed photothermal effects changed the intracellular Ca2+ levels and similar effects was observed with helium-neon and near-IR radiation.16 Moreno et al. found only sites along the beam path were activated and they suggested the neural responses were arisen from direct interactions between incident radiation and neural tissue, rather than the mechanical stimulation of HCs.17
Besides, there are also researchers supporting an optoacoustic effect as the underlying mechanism. Teudt et al. recorded the cochlear microphonic response and compound action potentials (CAPs) in rats with radiant wavelengths between 1850nm and 2120nm at a pulse duration of 100μs. Under conditions of thermal confinement, laser beams generated a pressure wave to which cochleae responded.3 Through widening the range of optical wavelengths from 420nm to 2150nm with pulse duration of 3–5ns and changing beam delivery locations, Schultz et al. determined that the most dominant stimulation effect was optoacoustic in nature. Optoacoustic stimulation depended on the water and hemoglobin absorption, and it was related to the functionality of the HCs.18 Later on, Schultz et al. continued applying hydrophone measurements in a water tank and measured compound action potentials (CAPs) in acutely deafened guinea pigs to further study the optoacoustic effect.19 Widening the pulse duration from nanosecond to millisecond, Kallweit et al. measured the pressure evoked by pulsed laser stimulating a water cylinder and the corresponding signal shapes of laser absorption in intracochlear optical stimulation of guinea pigs. They proposed an optoacoustic effect as the basic mechanism of INS.20
Photothermal and optoacoustic effects are two highly debated mechanisms for infrared neuron stimulation of the auditory nerve. In the photothermal mechanism, tissue absorbs laser energy and undergoes transient heating, which activates heat-sensitive ion channels in the tissue and evokes a response by the nerve. In the optoacoustic mechanism, some substance in the tissue (e.g., water) absorbs energy from the pulsed modulated laser, leading to a temperature change and corresponding tissue volume expansion and shrinkage. These changes in tissue volume radiate sound waves outwards and evoke the auditory path. Thus, essential steps towards distinguishing photothermal from optoacoustic mechanisms include verifying the existence of outer cochlear HCs and measuring the photoacoustic pressure under conditions of acute and chronic deafness.
In this paper, we used a pulsed laser of 980nm to stimulate cochleae of guinea pig models of acute and chronic deafness. To verify the effects of deafness, the stretched cochlear preparations were obtained and observed by fluorescence microscopy. To inspect the sound pressure in ambient air and water, we used a microphone and hydrophone, respectively. Our results show chronic deafness caused by the injection of streptomycin sulfate was effective in damaging outer HCs of the cochlea. We found switch response effect in vitro and in vivo experiments and we proposed that the photoacoustic effect could evoke OCAPs from the auditory nerve.
2. Materials and Methods
2.1. Schematic diagram
Figure 1 depicts a schematic diagram of the acoustic and infrared neuron stimulation. For acoustic stimulation, a speaker (SPA2380/93, Philips Investment Co., Ltd., Shanghai, China) was placed close to the ear of a guinea pig to present a tone burst, which was triggered synchronically by a physiological signal acquisition system (RM6240C, Chengdu Instrument Factory, China). For infrared neuron stimulation, a current source controller (LSR-PS-FA, Ningbo Yuan Ming Laser Technology Co., Ltd., Ningbo, China) was used to modulate the output power of the laser (980nm, LSR465CP-FC-3W, Ningbo Yuan Ming Laser Technology Co., Ltd., China). Pulse duration was modulated by a microcontroller unit (MCU, MC9S12XS128, Freescale Semiconductor, Inc.). Laser output was coupled to a 200μm-diameter optical fiber fixed on a micromanipulator (MP-225, Sutter Instrument Co., Beijing, China). The end of the optical fiber was oriented toward SGNs in Rosenthals canal in the basal turn. Synchronically triggering and data acquirement were processed in RM6240C.
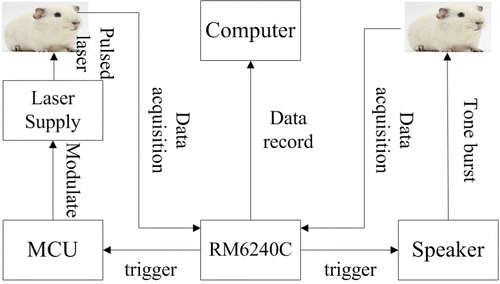
Fig. 1. A schematic diagram of the acoustic and infrared neuron stimulation. The pulse width was modulated by a microcontroller unit. The laser output was coupled into a 200μm-diameter optical fiber. The speaker is to give acoustic stimuli and the RM6240C is to acquire all the CAPs.
2.2. Animal model of deafness and surgery
Guinea pigs were under good care and used in accordance with the guidelines of the Administrative Committee on Animal Research at the Graduate School at Shenzhen, Tsinghua University. Guinea pigs of either gender were given daily intraperitoneal (i.p.) injections of streptomycin sulfate at 150mg/kg body weight (n=5, chronic deafness group) or physiological saline at 2.5mL/kg body weight (n=5, acute deafness group) for 4 weeks. Then the surgery was performed for all animals following the procedure.21 Each animal was positioned on a heating pad (BORO, BR Pet Products Co., Ltd., China), and body temperature was maintained at 38 C. Anesthesia was initiated by injection of 20% ethyl carbamate (6ml/kg body weight), followed by maintenance doses of approximately 3ml/kg body weight given every hour after paw pinch assessment.The mastoid bone beside the skull was exposed. An approximately 2mm-diameter hole was drilled in the mastoid bone and opened to allow access to the cochlea. Acoustic CAPs (ACAPs) were measured after removing the tympanic membrane and ossicular chain of animals. For the acute deafness group, 10μl of streptomycin sulfate solution were instilled in the cochlea through the round window.
2.3. Stimulation and signal acquisition
The guinea pigs were transferred to an anechoic chamber after surgery. A silver electrode (XF100, Chengdu Instrument Factory, China) was placed at the promontorium tympani near the round window to serve as a recording electrode. A reference electrode was clamped to the auricular skin, and a ground electrode was inserted into the dorsal skin. The speaker presented a tone burst at a rate of 0.5Hz as acoustic stimuli. Optical stimuli were generated by a 980nm diode laser with pulse durations ranging 1 to 20ms. ACAPs and optical CAPs (OCAPs) were measured by an RM6240C at a 100-kHz sampling rate. To reduce random error, a final CAP signal was obtained from the average of 20 measurements of CAP signals.
2.4. Stretched preparations and SGN detection
After the CAP measurements completed, the guinea pigs were given an overdose injection of 20% ethyl carbamate (200ml/kg body weight), the neurocranium was dissected, and the cochlea was removed within 3min. Then we immersed the cochlea in 4% paraformaldehyde for 2h at room temperature, followed by 10% ethylenediaminetetraacetic acid for decalcification at 4 C for 8h. Later we washed the cochlea in a 60mm cell culture dish with phosphate-buffered saline (PBS, pH 7.4, 5min X 3 times, 40 speed slow shake shaker). PBS was removed, and a dilute solution of Hoechest stain (Hoechest: PBS = 1: 2000) was added to stain the cochlea for 3min. Stain was removed, and the above washing process was repeated. Cochlear tissue was placed on a slide with 25 l of mounting medium and mounted with a coverslip. Stretched cochlear preparations were observed under a fluorescence microscope (Leica, DM400B/DFC480, Germany) stimulated with blue light (340–380nm).
2.5. Microphonic and hydrophonic measurements
All microphonic and hydrophonic experiments were performed using equipment and software from Brüel & Kjær Sound & Vibration Measurement A/S (Denmark). A microphone (4954-A) and amplifier-connected hydrophone (8103) were used to measure the pressure of optoacoustic stimulation in ambient air and a water tank (size: 10×10×10cm3), respectively. Microphone and amplifier were linked to a portable PULSE system (3560 C). An optical fiber was placed in a 90∘ orientation to the sound vibration sensors at a distance of about 2mm. The placement of microphone and the fiber is the same as Teudts program. A peak power of 2.3 W was used for the experiment. The PULSE 14.0 system read and displayed the signal.
3. Results
3.1. Comparison of ACAPs between chronic and acute deafness models
Figure 2 shows ACAPs measured from a guinea pig in the chronic deafness group and a normally hearing guinea pig without removing the tympani membrane and osiccular chain as a control. The ACAP threshold for the animal in the chronic deafness group was about 95dB, compared to less than 58dB for the control group. In other words, chronic deafness affected the auditory responses of guinea pigs.
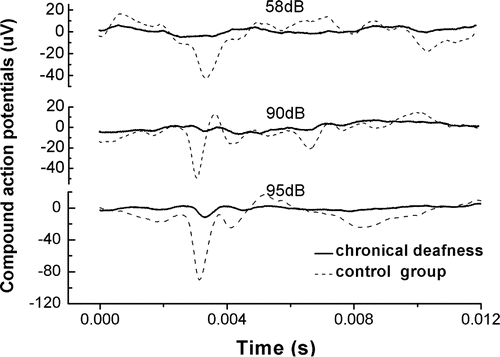
Fig. 2. ACAPs measured from a chronically deafened guinea pig (chronical deafness) and a normal guinea pig (control group). The sound pressure of short pure stimuli from bottom to top is 95, 90, 58dB, respectively. For chronically deafened guinea pig, the hearing threshold is about 95dB while the normal one is less than 58dB.
Figure 3 shows acoustic threshold elevation of the animal in the acute deafness group was about 70dB. Thus, the process for inducing chronic deafness was more effective at causing hearing loss than the process for acute deafness.
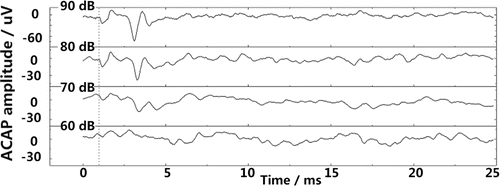
Fig. 3. ACAPs measured from an acutely deafened guinea pig. The sound pressure of short pure stimuli from bottom to top is 60, 70, 80, 90dB, respectively. The dotted line on the left represents the starting time of acoustic stimuli. There is an obvious ACAPs observed only when sound pressure is about 70dB.
3.2. SGN survival detection
Results of SGN detection in stretched cochlear preparations under fluorescence microscopy are shown in Fig. 4. Nuclei of live cells absorbed Hoechest solution and reflected blue fluorescence under stimulation with light at 340–380nm. Guinea pigs in the chronic deafness group showed missing nuclei, karyolysis, and nuclear swelling, indicating that a portion of the outer HCs were damaged or lost (Fig. 4(a)). By contrast, stretched cochlear preparations in the acute deafness group revealed nearly intact outer HCs (Fig. 4(b)).
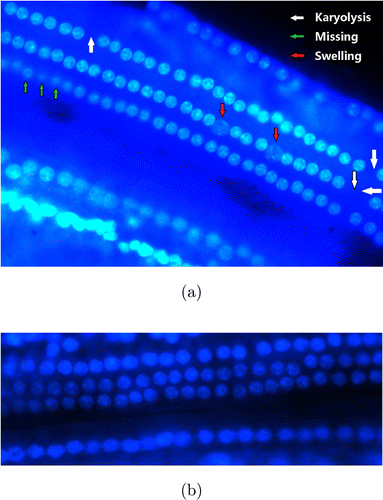
Fig. 4. The cochlear stretched preparations observed under a fluorescence microscope. The three bright bead chains in the upper left corner are the outer hair cells. (a) A cochlear specimen of a chronically deafened guinea pig. White arrows point to the position of nucleus missing, green arrows point to the karyolysis and red arrows point to the swelling nucleuses and (b) A cochlear specimen of an acutely deafened guinea pig. Outer hair cells were almost intact.
3.3. OCAPs measurement
Figures 5 and 6 depict the differences of OCAPs between the chronic and acute deafness groups, in response to pulse durations from 1 to 20ms at constant peak power (870mW). OCAPs can be seen in both pictures. For guinea pigs in the chronic deafness group, OCAP amplitudes were lower than those of the acute deafness group. We observed the rare phenomenon of a second OCAP when the pulse duration exceeded 2ms. The second OCAP appeared around the stopping time of the pulse. We call this phenomenon the switch response effect. Amplitudes of the second OCAPs were larger than amplitudes of the first OCAPs for pulse durations longer than 3ms (Fig. 5(a)).
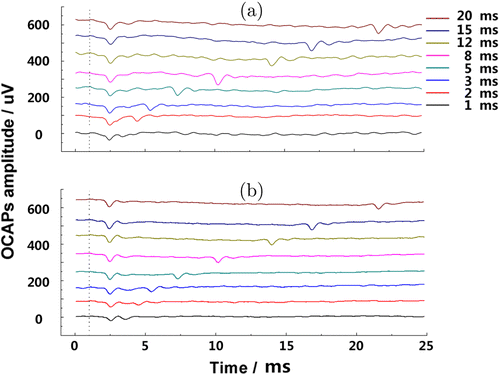
Fig. 5. OCAPs measured from the chronically. (a) acutely and (b) deafened guinea pigs. The dotted line on the left represents the starting time of acoustic stimuli. Different colors stand for different pulse duration under the same radiant exposure. OCAPs are found in each line and some of them emerge two OCAPs when pulse durations are more than 2ms.
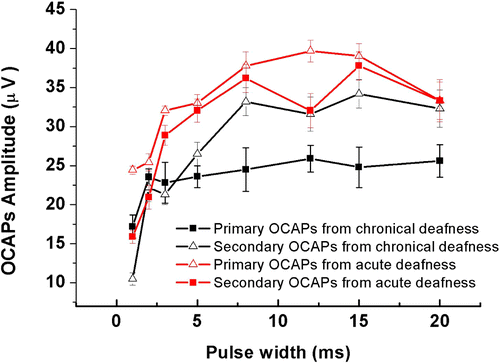
Fig. 6. Statistic results of OCAPs amplitudes obtained upon different pulse width (1, 2, 3, 5, 8, 12, 15, 20ms). The red lines and black lines stand for acutely and chronically deafened guinea pigs respectively, n=5.
3.4. Photoacoustic stimulation
We measured the optoacoustic effect in ambient air using a high-sensitivity microphone under pulsed laser durations of 20ms. We observed a bipolar pulse with a peak-peak amplitude about 40Pa (Fig. 7(a)). We also measured the optoacoustic effect in a water tank using pulsed laser durations of 20ms (Fig. 7(b)). The similar bipolar pulse waves emerged during the pulse time.
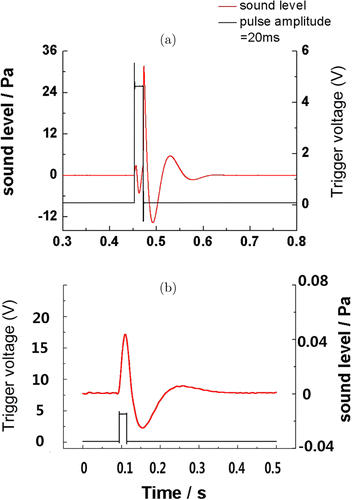
Fig. 7. Optoacoustic effect measurement in ambient air. (a) in a water tank and (b) The red color lines represent sound signals and the black ones represent pulsed laser stimulation. Stimulation pulse time is 20ms.
4. Discussion
There has been a lot of recent researches about the mechanism of optically evoked CAPs. Several groups have proposed a photothermal mechanism, in which the accumulation of heat leads to alterations of ion channel activity, resulting in the auditory response.22–26 In contrast, our experiment results indicate that the optoacoustic effect is the most likely mechanism for OCAPs. Some authors have tried to exclude the optoacoustic effect by chronically or acutely deafening guinea pigs,14,21 and thereafter evaluating the deafening effect by measuring the threshold with pure acoustic stimuli. In our experiment, guinea pigs in the chronic and acute deafness groups had obviously increasing acoustic thresholds of more than 30dB (Fig. 2). Nevertheless, fluorescence microscopy data revealed perfectly intact inner HCs. Some of the outer HCs were lost after induction of chronic deafness, whereas the process of inducing acute deafness did not seem to affect the HCs. We observed OCAPs in this experiment, which suggests that even the chronic deafness process could not completely damage the outer HCs. Residual outer HCs might still respond to the acoustic stimulation evoked through the photoacoustic effect.
A typical photoacoustic effect could be illustrated in Fig. 8 of which a bipolar pulse could be evoked by pulsed laser stimulation in liquid.27 Markus Sigrist and Fritz Kneubuhl studied the generation of laser-induced stress waves in liquids with a high-speed camera and special high-sensitivity stress transducers at nanosecond level and they observed a bipolar pulse. Paltauf, etc. also found a bipolar pulse in both theoretical calculation and experiment and the interval time between two peaks of the bipolar pulse related to the pulse duration of lasers.26
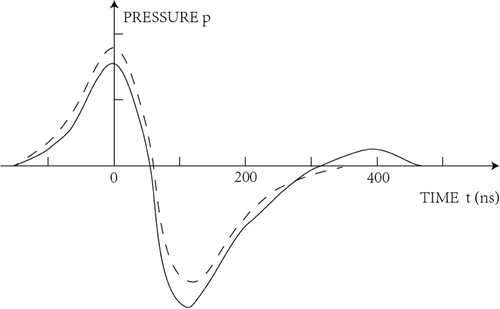
Fig. 8. Theoretical thermoelastic acoustic wave according to Markus Sigrists model (dashed cure) his experimental result (solid curve).
In our experiment, we observed a switch response effect when the pulse duration was increased to 3ms, with the appearance times of two OCAPs corresponding to the onset and offset times of the pulse. This phenomenon could hardly be explained by the photothermal effect. Similarly, the switch response effect also occurred when we used the microphone to measure the acoustic pressure of pulsed laser stimulation. One possibility is that after the pulse starts, the liquid absorbs energy and its volume expands, resulting in the first bipolar pulse. At the end of the pulse, the liquid energy disperses and the volume decreases, producing another bipolar pulse. Amplitudes of the second OCAPs were larger when pulse duration exceeded 3ms, consistent with the results shown in Fig. 5.
Previously many research groups like Izzo and Richter et al. applied INS with laser durations from 5ns to 300μs and they have not reported this switch response effect. There are two possible reasons for this discrepancy from our findings. One possibility relates to the pulse duration necessary to produce two bipolar pulses. We found that there was a threshold pulse duration (>1ms) for observing the switch response effect. Most of the previous researchers focused on the pulse width of microsecond or even nanosecond level. The second possibility relates to the 1–2ms latency of the guinea pig auditory system to respond to sound or laser stimuli.13,21 Pulsed laser within a pulse duration of less than 2ms was unlikely to evoke two OCAPs. Our previous study also shows that when stimulation rate is over 400Hz (2.5ms), there would be only one CAP in the auditory response.20 Therefore, we proposed photoacoustic effect could invoke auditory response in infrared neuron stimulation.
5. Conclusions
Injecting streptomycin sulfate to induce chronic deafness was effective in damaging outer HCs of the guinea pig cochleae. The switch response effect in measurements of pressure for pulses in vitro and OCAPs in vivo indicated photoacoustic effect could invoke auditory response in infrared neuron stimulation.
Acknowledgments
This project was supported by grants from the Nature Science Foundation of China (Nos. NSFC81401539 and NSFC31271056) and the projects in the Shenzhen Medical Engineering Laboratory For Human Auditory-equilibrium Function.