The Application of Inorganic Optical Nanoprobes in Bacterial Infection
Abstract
Bacterial infection is an acute infection caused by pathogens or conditional pathogens, which leads to severe disease and even death. It has become a significant reason for diseases and deaths worldwide. Therefore, rapid and precise detection, diagnosis, and treatment in the early stage are the key to deal with bacterial infections. Over recent years, along with the advances in biomaterials and nanotechnology, numerous nanomaterial-based multifunctional probes have been extensively explored in the biomedical field. Because of their excellent optical properties, inorganic optical nanoprobes are used to rapidly detect bacterial infection in the early stage and show excellent antibacterial properties, which has a great application prospect in antibacterial therapy expected to reduce the risk of bacterial infection. In this mini-review, we generally overviewed and summarized recent progress on inorganic nanoparticle-based optical imaging techniques as a platform to construct functional theranostics for the efficient treatment of bacterial infections. The opportunities and challenges in the application of fluorescent optical nanoprobes are prospected.
1. Introduction
For the past few decades, bacterial infections have emerged as a serious threat to people’s health, with nearly a quarter of all deaths worldwide attributed to diseases caused by bacterial infections.1,2,3 However, in most recent years, as a result of the abuse of antibiotics, the appearance of drug-resistant bacteria and even superbugs has led to the re-emergence of infectious diseases that were once effectively controlled.4,5,6,7 Diagnosis of bacterial infections is the most direct and effective way to curb the misuse of antibiotics. However, because of the low sensitivity of current tests for bacterial infections and the difficulty of distinguishing bacterial inflammation from aseptic inflammation with conventional tests, bacterial infection diagnosis is not used as the primary pre-drug test in clinical medicine.8,9 Therefore, the development of efficient techniques for diagnosing bacterial infections is one of the most pressing clinical issues.
Optical imaging technology usually uses exogenous fluorescence or light-emitting probe to mark specific areas, while sensitive optical detectors are used to collect signals and generate images. After being developed rapidly over the years, this technology has been widely used in disease diagnosis and biosensors.10,11,12,13,14 Based on the concept of optically guided therapy, the development of fluorescent probes capable of fast identification and treatment in the early stages of bacterial infections is expected to reduce the risk of bacterial infections. Recently, the rapid advances in nanotechnology have further provided new therapeutic and diagnostic avenues for bioapplication, and a variety of nanoparticulate multifunctional probes have been widely used in the biomedical field.15,16,17,18 Some research articles have recently been published on the use of optical probes based on organic nanomaterials to detect bacteria.19,20,21,22 And there are also several excellent reviews summarizing the use of nonnanoparticle-based optical probes in bacterial infections,23,24,25,26,27 which is not the focus of this paper. This review here presents several types of inorganic nanoparticle-based optical imaging techniques recently used in bacterial infections with challenges and perspectives.
2. The Properties of Inorganic Optical Nanoprobes
Overall, inorganic nanoparticles offer many advantages over organic nanoparticle formulations, such as low cost, facile manufacture, broad surface conjugation possibility, good biocompatibility, long body retention time, and the ability to control affinity and pharmacokinetics better. While with the rapid advances in nanotechnology, inorganic nanoparticles serve an essential role in many fields, such as biosensors and bioimaging, due to their small sizes, high surface area-to-volume, as well as distinctive optical features. For example, semiconductor quantum dots (QDs) show tremendous potentials in bioimaging owing to their high fluorescence quantum yields and wide emission ranges. Gold nanoparticles (AuNPs) demonstrate vast applications in the detection of small analytes and biomolecules through surface modifications. In the following sections, we briefly outlined the properties of different material types of inorganic nanomaterials according to their characteristics and listed some recent research results focusing on these materials for bacterial imaging.
3. Quantum Dots
QDs are “zero-dimensional” fluorescent nanoparticles consisting of a certain number of atoms or molecules, usually constituted of group II–VI or III–V elements, with a particle size typically within 100 nm.28,29 As a semiconductor fluorescent nanoprobe, quantum dots exhibit excellent spectral properties and optical stability due to their unique quantum size effect and surface effect. For example, compared to conventional organic dyes, the excitation spectrum of quantum dots is broader and continuous, while the emission spectrum is narrower and symmetrically distributed.30,31 This spectral property reduces spectral overlap and makes simultaneous multiple fluorescence imaging possible. On the other hand, QDs with diverse sizes could be excited by light of a single wavelength and emit different colors.32,33 This property makes it possible to construct fluorescent probe systems for simultaneous multicomponent detection. Besides, the ease of surface modification allows QDs to be coupled to a wide range of biomolecules, resulting in good biocompatibility.
Bruchez et al. first applied QDs as a fluorescent nanoprobe in biological staining and diagnosis in 1998, achieving the goal of using it as a specific biomarker and setting a milestone for the research of QDs in the biomedical field.34 Nowadays, there are various QDs that have been used to label bacteria strains. For example, Yu et al. developed an immunochromatography test strip based on CdTe/CdS QDs as fluorescent markers, which were further coupled with polyclonal antibodies.35 The antibody–QDs conjugation detected E. coli O157:H7 rapidly with a limitation of 104 CFU/mL. Using magnetic nanoparticles (MNPs) and multiring magnetic field generator, a novel fluorescent biosensor based on immune QDs was developed by Xue et al. [Fig. 1(a)].36 In this system, the E. coli cells are initially recognized and captured by MNP under the magnetic force, forming a complex of MNP–bacterium. It then reacts with immune QDs to create a triple complex of MNP–bacterium–QDs. The whole complex can be trapped and measured by a portable optical system to generate the fluorescence intensity reading of bacterial cells. As they report, the ultra-sensitive sensor can not only detect up to 14 CFU/mL of cells, but also be utilized to detect large volumes of bacteria up to 10mL. Heavy metal-free ZnCuInSe QDs were proposed by Geng et al. for the fast differentiation of Gram-positive and Gram-negative bacteria.37 Driven by the structure of Gram-positive bacteria, these ZnCuInSe QDs are able to effectively bind to S. aureus and give discernable color that could be observed by naked eyes. Meanwhile, due to the typical fluorescence response of ZnCuInSe QDs, the accurate quantification of bacteria could be obtained by a photoluminescence system with a range of 103–1011 CFU/mL. Recently, Yan et al. provided a new rapid method to discriminate Gram-positive bacteria, called three excitation peaks and single-color emission carbon quantum dots (T-SCQDs) [Fig. 1(b)].38 These QDs also can track the bacteria as long as 24h via one-step staining. In addition, the T-SCQDs enable selective discrimination of colony types according to fluorescence intensities and the visual differentiation of bacteria from infected A549 cells by confocal fluorescence microscopy.
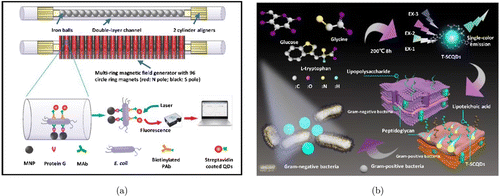
Fig. 1. (a) The schematic illustration of a QDs-based supersensitive biosensor and its working mechanism. Reproduced from Ref. 36 (Copyright 2018 Elsevier B.V.). (b) Synthetic routes for T-SCQDs and the utilization in peptidoglycan-targeted recognition of Gram-positive bacteria. Reproduced from Ref. 38 (Copyright 2021 American Chemical Society).
Along with their unique optical properties, the QDs also exhibit excellent antimicrobial properties, which are associated with the induction of free radicals, disrupting cell walls/membranes, and blocking gene expression. QDs present exclusive advantages treating bacterial infections. Zhang et al. obtained an efficient antimicrobial composite (GQDs@AgNPs) by coating graphene quantum dots (GQDs) onto the surface of AgNPs, which demonstrated promising synergistic antibacterial performance against S. aureus and E. coli.39 Due to the releasing of silver ions, the GQDs@AgNPs composite can change the cell-membrane permeability, damage DNA, decrease dehydrogenase activity, and produce oxidative stress to kill the bacteria. Alternatively, GQD can inactive bacteria by generating more reactive oxygen species (ROS) through the disproportionation of silver ions. This synergistic antibacterial mechanism leads to the sterilization and death of bacterial cells.
Although some progress has been made in applying QDs technology to bacterial infections, there is yet a long journey to be made to bring QDs into use in clinical trials. In addition to concerns about the biosafety of QDs, there are a number of critical questions that need to be answered; for example, the distribution and functional efficacy of QDs in deeper tissues of the human body, and how to distinguish between QDs-labeled biomolecules and the body’s biomolecules. In other words, QDs have not been developed for a long time, but their emergence has dramatically enriched the family of fluorescent probes and promoted the development of fluorescent probe technology, which has been rapidly applied in the field of life science research. We have reasons to believe that developing QDs will have a broader application and contribute to the development of life sciences.
4. Gold Nanoparticles
AuNPs are composed of an inorganic gold core and an outer monolayer of organic molecules. According to the different shapes formed during the preparation of AuNPs, they can be divided into nanospheres,40,41,42 nanorods,43,44,45,46 nanocages,47,48,49 nanostars,50,51,52,53 and “core–shell” nanostructures.54,55,56 AuNPs with different structures often have significant differences in optical, electrical, and catalytic properties.57,58,59,60 As one of the earliest discovered metal nanomaterials, AuNPs are sensitive to optical properties and easy to functionalize with various ligands. According to the properties of the object to be detected, the spectral probe based on AuNPs can be designed and synthesized purposefully. The construction of different chemical or biological probes using AuNPs has become essential in dealing with bacterial infections.
At present, AuNPs is widely used in bacterial imaging detection. Jin et al. developed a fluorescence resonance energy transfer (FRET)-based platform for rapid, ultra-sensitive, and specific bacterial detection [Fig. 2(a)].61 The AuNPs (acceptors) were covalently connected to aptamers in this platform, while the upconversion nanoparticles (UCNPs; donors) were functionally conjugated with the corresponding cDNA. The overlapping of spectra between the absorption of the AuNPs and the fluorescence emission of the UCNPs allowed FRET to occur upon hybridization of the cDNA and the aptamer, thereby leading to quenching of the upconversion fluorescence. When bacteria are present, the aptamer preferentially bound to the bacteria to form a three-dimensional structure, thus dissociating the UCNPs–cDNA complex from the AuNPs–aptamers, which resulted in the recovery of upconversion fluorescence. Employing this sensor, they successfully detected E. coli within 5–106 CFU/mL, achieving an ultra-low threshold of 3 CFU/mL detection. A highly sensitive fluorescence nanobiosensor was developed by Elahi et al. for sensing the Shigella species [Fig. 2(b)].62 In this biosensor, two DNA probes were engineered and anchored to the surface of the AuNPs as signal reporters. Another DNA probe was immobilized on the surface of iron nanoparticles to separate the target DNA. After being added with the target DNA, the whole complex was isolated under magnetic field. The fluorescence probe was released on the surface of AuNPs, which could be read by fluorescence spectrophotometry. The detection limit was reported as 90 CFU/mL, suggesting this nanobiosensor has excellent sensitivity and specificity. Zhang et al. proposed a three-in-one composite that combines a novel bacitracin-based flocculant, the surface-enhanced Raman scattering (SERS) tags, and modified Au@AgNPs.63 In this system, the amended Au@AgNPs capture the bacteria and cause them to cluster around the Au@AgNPs, creating rich “hot spots” and robust Raman signals. Using this triple system, they demonstrated clear fingermarks of different bacterial species in Raman mapping, such as P. aeruginosa, E. coli, E. faecalis, and B. cereus.
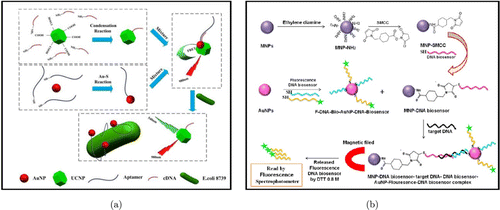
Fig. 2. (a) Schematic diagram of an FRET sensor based on UCNPs for fast and ultra-sensitive bacterial detection. Reproduced from Ref. 61 (Copyright 2016 Elsevier B.V.). (b) Schematic diagram and working mechanisms of DNA-based nanobiosensor. Reproduced from Ref. 62 (Copyright 2019 Elsevier B.V.).
In addition to detecting bacteria, AuNPs also have therapeutic effects on bacterial infections simultaneously. For example, Du et al. presented an AuNS–Apt–Cy probe with gelatinase-responsive ability for the insitu near-infrared imaging and local photothermal therapy of methicillin-resistant S. aureus (MRSA) infections [Fig. 3(a)].64 The nanoprobe is based on gold nanocolumns functionally characterized with MRSA-recognizable inducers and gelatinase-reactive linkers (CPLGVRG). This AuNS–Apt–Cy nanoprobe enables the localization and accumulation of MRSA. Due to the overexpressed gelatinase in MRSA-infected microenvironments, the CPLGVRG was cleaved, leading to turn on NIR fluorescence; and accompanied with the disruption of bacterial cell wall and membrane, the antimicrobial photothermal therapeutic effect can be achieved. Based on the discrimination of bacterial virulence factors, LecA and LecB, Zhang et al. reported glycoconjugate-based AuNPs imaging and therapeutic strategies for P. aeruginosa [Fig. 3(b)].65 Both Fuc–AuNPs (targeting LecB) and Gal–AuNPs (targeting LecA) are loaded with the fluorescent moiety dicyandiamide-4H-pyran in a noncovalent manner to obtain a highly selective fluorescent imaging agent for the observation of bacteria. Using the phototherapeutic properties of AuNPs, 600 nm light irradiation activated Fuc–AuNP/Gal–AuNP, providing photothermal and photodynamic therapy effects and facilitating the release of the loaded antibiotic, Ceftazidime. Combining the phototherapeutics and chemotherapeutics, both the Ceftazidime-loaded Fuc–AuNP and Gal–AuNP were capable to completely eradicate biofilms caused by P. aeruginosa. Recently, Hao et al. prepared multifunctional platforms containing NIR light-responsive vancomycin-doped Prussian blue nanoparticles (PB-VANNPs)NPs) for dual-mode bacterial detection and real-time sterilization.66 PB-VANNPs are capable of binding to S. aureus to create PB-VANNPs–bacteria complexes. Upon irradiation, the suspension can trigger the release of oxygen from perfluorohexane, which could be detected with the portable manometer sensitively, with a detection limit of 1 CFU/mL. Alternatively, the complex precipitate would be captured by thermal imaging, recording the same detection limit; meanwhile, the bacteria were effectively sterilized with the local temperature elevation during the process (99.8%).
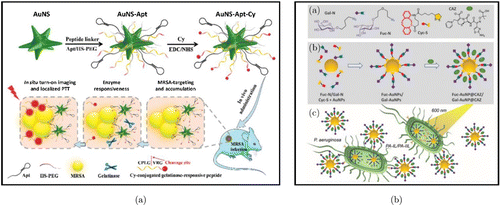
Fig. 3. (a) Schematic illustration of AuNS–Apt–Cy optical nanoprobes for insitu NIR imaging and localized PTT of MRSA infections. Reproduced from Ref. 64 (Copyright 2020 The Royal Society of Chemistry). (b) (a) Critical blocks for the fabrication of Fuc–AuNPs and Gal–AuNPs; (b) schematic diagram of self-assembly procedures and co-encapsulation of CAZ to form Fuc–AuNP@CAZ and Gal–AuNP@CAZ; and (c) schematic representation of the targeted lectin approach with specific access to P. aeruginosa and simultaneous release of antibiotics and ROS/heat production in the light. Reproduced from Ref. 65 (Copyright 2020 The Royal Society of Chemistry).
The advancement of bacterial biosensors based on AuNPs has progressed considerably in the last decades. For example, selective recognition of bacteria through various surface modification strategies has been achieved to label a particular bacterium specifically in the detection. However, significant challenges remain to be tackled before clinical application can proceed. The stability of metal nanoparticles is one of the first points to be considered. In complex biological systems, metal nanoparticles may form nonspecific aggregates, leading to false positive or negative signals. In addition, clarification of the photoluminescence mechanisms of AuNPs, such as fluorescence resonance energy transfer or plasmon-enhanced photoluminescence, is essential for the design of biosensors.
5. Carbon Nanotubes
Carbon nanotubes (CNTs) have a large Stokes shift and have considerable functionalization targeting agent flexibility, making them suitable to be exploited as a targeted optical fluorescence imager for cell, tumor, and vessel systems.67,68,69,70 Single-walled carbon nanotubes (SWCNTs) are becoming attractive candidates as fluorophores for second-window near-infrared imaging, which can provide a better penetrability in biological tissues than visible organic dyes and the first-window near-infrared.71,72,73,74 For example, Bardhan et al. described M13 bacteriophage-functionalized SWNTs (M13-SWNTs), which could distinguish between F’-positive (JM109) and F’-negative (DH5-α)α)E. coli strains.75 In a further extension of this work, the SWCNTs were linked to an antibody to target the F’-negative S. aureus, which successfully demonstrated the diagnosis of S. aureus intramuscular infections and infective endocarditis, accompanied by a several-fold boost in fluorescence intensity [Fig. 4(a)]. Recently, Hicks et al. described an electrochemical biosensor consisting of SWCNTs immobilized on an indium tin oxide surface which was functionalized with an osmium-based composite.76 The sensors were found to have a low LOD for hydrogen peroxide and a fast response, resulting in an unprecedented detection at the temporal level, which was previously unseen in the response to bacterial menaces. Moreover, due to the structure, positively charged ends, physical antimicrobial mechanism, and highly efficient ROS production, CNTs are considered as potential novel platforms of antimicrobial drug delivery system. Recently, combined with the drug loading capability and imaging ability, Khazi-Syed et al. employed SWCNTs to deliver antibiotics and track S. epidermidis in mammalian cells.77
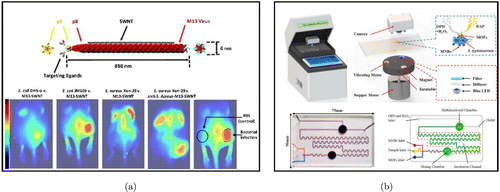
Fig. 4. (a) Structure of the SWNT probe. M13 is a bacteriophage with typical dimensions of 880nm in length and 6 nm in diameter. Representative images of mice infected with different bacteria strains. Reproduced from Ref. 75 (Copyright 2014 Macmillan Publishers Limited). (b) The prototype and structure of a microfluidic biosensor and its microfluidic chip. Reproduced from Ref. 84 (Copyright 2021 Elsevier B.V.).
6. Other Organic Optical Nanoprobes
Metal–organic frameworks (MOFs) are novel medical relevant nanomaterials that have recently attracted worldwide attention. Numerous applications for MOFs have been presented, including catalysis, isolation, sensing, and pharmaceutical delivery.78,79,80,81,82 As a result of their characteristics, such as large surface area, biodegradability, and biocompatible structure, they are suitable to be designed as optical nanoprobes and nanocarriers with particular promises, such as cell-specific targeted delivery, cross-barrier transport, and visualization of delivery sites. Gupta et al. reported an electrochemical biosensor which is based on Cu–MOF for the detection of E. coli.83 This novel MOF/PANI-based platform exhibited high sensitivity (2 CFU/mL) to detect E. coli in a terse reaction time (nearly 2 min) and showed high-level selectivity in the presence of other common bacterial strains. A microfluidic biosensor was developed by Qi et al. for fast, sensitive, and automated detection of Salmonella [Fig. 4(b)].84 The target Salmonella species are enriched and blended with immune magnetic nanobeads (MNBs) and MOFs to give an MNB–Salmonella–MOF complex, which is subsequently used to catalyze o-phenylene diamine, which is colorless, and H2O2, producing yellow 2,3-diaminophenol. And finally, it is read by an App to present the bacterial concentration.
The fluorescent liposome represents a kind of nano-based vesicles, as such delivery platform could be surface modified or encapsulated with fluorescent molecules to act as optical nanoprobes. Besides acting as drug delivery systems and bacteria-labeling probes, the ease of surface modification and capability of loading molecules allow liposomes to perform functions such as actively targeting bacteria and absorbing bacterial toxins. Our group previously reported maltohexaose-decorated bacteria-responsive nanoliposomes, loaded with a potent sonosensitizer, called purpurin18.85 These nanoliposomes can target the site of bacterial infection specifically and discriminate accurately between infected lesions and sterile inflammation or cancer through the highly selective optical signals in mice [Fig. 5(a)]. Furthermore, we developed the engineered cell-membrane nanovesicles, which presented an antibody that can neutralize the αα-toxin on the nanovesicles’ surface, enabling to capture virulence more potently.86 This sono-immunotherapeutic platform also provided invivo optical imaging and insitu magnetic resonance imaging to visualize the treatment progression [Fig. 5(b)].
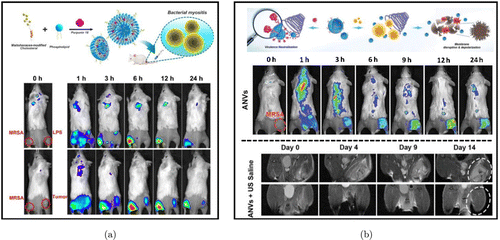
Fig. 5. (a) Scheme illustration of MLP18 nanoliposomes (upper). Representative fluorescent images of MRSA-infected mice after administration of MLP18 (below). Reproduced from Ref. 85 (Copyright 2019 American Chemical Society). (b) Schematic diagram of the antivirulence and antimicrobial mechanisms of ANVs (upper). Representative fluorescent images of MRSA-infected mice after tail vein administration of ANVs (middle). Representative magnetic resonance photos of the MRSA-infected mice within 14 days after giving the ANVs (below; the circle indicates the MESA-infected area). Reproduced from Ref. 86 (Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA).
7. Challenges
Optical nanoprobes are semiconductor quantum dots or fluorescent groups that can emit fluorescence introduced into organic or inorganic nanoparticles through encapsulation, covalent linking, supramolecular assembly, etc., and allow the nanoparticles to take on the detection, labeling, and even therapeutic functions of fluorescent dyes. Compared to conventional fluorescent probes, optical nanoprobes offer higher brightness and stability and easier dispersion in the aqueous phase and biocompatibility. However, as with other nanoparticle-based systems, nanooptical probes can confront a sophisticated list of biological obstacles that seriously restrict site-specific bioavailability and prevent the realization of appropriate therapeutic effects, such as mononuclear phagocyte system (MPS), nonspecific biodistribution, cellular internalization, etc. The first and foremost limitation comes from the MPS, which enables the elimination of nanoparticles through phagocytosis. Thus, in the case of common bacterial infections, in order to prolong nanoparticles’ retention to reach the infected site and achieve pharmaceutical effects, various strategies have been adopted in the design of nanoparticles to make them less susceptible to recognition by the MPS, such as coating nanoparticles with PEG and self-identifying markers to render prolonged circulation in the bloodstream and reduced uptake by the MPS. However, another issue faced by inorganic optical nanoprobes while prolonging their circulation time in vivo is their potential toxicity and side effects. Therefore, in order to systematically assess the toxicity and side effects of inorganic optical nanoprobes for future clinical translation, their biodistribution and metabolic toxicity in different organs, as well as the corresponding acute toxicity, chronic toxicity, and genotoxicity, in different animal models should be examined first.
8. Conclusion and Perspectives
This review aims to summarize and discuss some of the most relevant topics related to the detection of bacterial infections. We provide information on the various types and approaches to detecting bacteria using quantum dots, gold nanoparticles, carbon nanotubes, as well as different organic nanoparticles such as metal–organic frameworks and nanoliposomes. The use of nanoparticle-based optical probes may further advance particularly sensitive and specific assays for bacterial diagnosis. Currently, nanoprobe is still at the beginning of its development, and there are no such optical nanoprobes licensed for clinical usages. However, nanoparticle-based agents have shown tremendous promise in all aspects of biomedicines. We can be confident that with further improvements in nanotechnology, precise control of the scale of nanoparticles, and the increasing refinement of the means of functionalizing particles, to a large extent, nanoparticles will be able to meet the requirements of biosensors, biological probes, and clinical theranostics.
Conflict of Interest
The authors declare that there are no conflicts of interest relevant to this paper.
Acknowledgments
The authors are grateful for the support of the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (81925019, 81422023, and U1705281), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019), and the Program for New Century Excellent Talents in University, China (NCET-13-0502).


