Surface-enhanced Raman spectroscopy of serum predicts sensitivity to docetaxel-based chemotherapy in patients with metastatic castration-resistant prostate cancer
Abstract
Docetaxel-based chemotherapy, as the first-line treatment for metastatic castration-resistant prostate cancer (mCRPC), has succeeded in helping quite a number of patients to improve quality of life and prolong survival time. However, almost half of mCRPC patients are not sensitive to docetaxel chemotherapy initially. This study aimed to establish models to predict sensitivity to docetaxel chemotherapy in patients with mCRPC by using serum surface-enhanced Raman spectroscopy (SERS). A total of 32 mCPRC patients who underwent docetaxel chemotherapy at our center from July 2016 to March 2018 were included in this study. Patients were dichotomized in prostate-specific antigen (PSA) response group (n=17n=17) versus PSA failure group (n=15n=15) according to the response to docetaxel. In total 64 matched spectra from 32 mCRPC patients were obtained by using SERS of serum at baseline (qq0) and after 1 cycle of docetaxel chemotherapy (qq1). Comparing Raman peaks of serum samples at baseline (qq0) between two groups, significant differences revealed at the peaks of 638, 810, 890 (p<0.05p<0.05) and 1136cm−1−1 (p<0.01p<0.01). The prediction models of peak 1363cm−1−1 and principal component analysis and linear discriminant analysis (PCA–LDA) based on Raman data were established, respectively. The sensitivity and specificity of the prediction models were 71%, 80% and 69%, 78% through the way of leave-one-out cross-validation. According to the results of five-cross-validation, the PCA–LDA model revealed an accuracy of 0.73 and AUC of 0.83.
1. Introduction
Prostate cancer (CaP) accounted for more than 1 in 5 new male cancers in the USA in 2020.1 Patients with CaP treated with androgen deprivation therapy (ADT) inevitably progress to metastatic castration-resistant prostate cancer (mCRPC) within 1–3 years of diagnosis. Unfortunately, mCRPC remains an aggressive disease of which prognosis is poor.2 How to extend their end-stage survival time is still an urgent problem for urologists to solve.
Docetaxel was officially approved as the first-line standard treatment of mCRPC by the US Food and Drug Administration in 2004.3 Moreover, for mCRPC patients with significant clinical symptoms and relatively good general status, docetaxel-based chemotherapy has been considered as the first choice in most cases. However, the results of TAX327 and SWOG9916 clinical trials showed that almost half of mCRPC patients were not sensitive to docetaxel chemotherapy, which meant numerous mCRPC patients could hardly obtain clinical benefits from docetaxel.4,5 In order to avoid meaningless treatment and painful side effects of chemotherapy, it is of great research value to explore the effective biomarkers or methods to predict sensitivity to docetaxel chemotherapy in patients with mCRPC.
Many previous studies have shown that some clinicopathological characteristics, blood biochemical indexes, and circulating tumor cell counts (CTCs) of patients before chemotherapy had certain predictive effects on the prognosis of mCRPC patients undergoing docetaxel chemotherapy. Armstrong et al., found that four independent risk factors were associated with the prognosis of mCRPC patients after docetaxel chemotherapy, including visceral metastasis, bone scan progression, significant pain, and anemia.6 Docetaxel might have more antitumor activity in patients with high-gleason-score diseases. Compared to 2.9 months for the whole patient cohort in the original TAX327 study, patients with Gleason score 7–10 could get an OS survival benefit of 4.4 months.7 De Bono et al., found that CTCs count had a strong predictive effect on the prognosis of mCRPC patients, and patients with CTC count greater than five had a significantly worse prognosis.8 However, these reported indexes or biomarkers often reflected the invasiveness of prostate tumors and general basic conditions of mCRPC patients before chemotherapy, instead of the response to docetaxel chemotherapy. Therefore, it was less-than-ideal to use these prognostic models as a reference to select the best treatment regimen for mCRPC patients.
As a noninvasive and informative technology, Raman spectroscopy can provide fingerprint-type molecular and chemical information of biological samples. Surface-enhanced Raman spectroscopy (SERS) is a special type of Raman spectroscopy, of which the signal can be dramatically augmented as much as 106–101414 by mixing the sample with silver or gold nanoparticles.9 SERS of serum samples, with its convenient and rapid detection process, gradually becomes an eye-catching and effective tool to detect the alters of various small molecules (such as glycogen, amino acids, peptide metabolites, lipids, and nucleic acids) generated by normal or tumor tissue in serum from the molecular level.10 Currently, serum SERS technology is mainly applied to the research on the early diagnosis of tumors, including prostate cancer, breast cancer, bladder cancer, colorectal cancer, oral cancer, and other tumors.11,12,13,14,15 Apart from that, a few studies reported that serum SERS could also predict tumor recurrence and monitor the effect of chemotherapy. Pan et al., utilized SERS to detect the preoperative serum of 102 patients undergoing radical prostatectomy for prostate cancer, and found that there was a significant difference in the relative peak intensity of 725, 1328, and 1447cm−1−1 between the biochemical recurrence group and the group without biochemical recurrence, p<0.01p<0.01; The sensitivity, specificity and accuracy of the Principal Component Analysis (PCA) model reached 65.8%, 87.5% and 79.4%, respectively.16 Gonzalez-Solis et al., successfully distinguished the different types of leukemia patients by serum SERS technology (sensitivity 100%, specificity 100%) and found that serum SERS could effectively monitor the chemotherapy response of leukemia patients.17 However, so far, little research has been reported to explain the role of serum SERS in predicting sensitivity to chemotherapy in solid tumor.
Therefore, our study investigated if serum SERS obtained from a cohort of mCRPC patients undergoing docetaxel chemotherapy could predict clinical response to docetaxel and eventually promote the application of accurate treatment in mCRPC.
2. Patients and Methods
2.1. Enrolled patients and evaluation of response to docetaxel chemotherapy
This retrospective study included patients with mCRPC who received docetaxel chemotherapy in our hospital from July 2016 to March 2018. Patients received 75mg/m2 of docetaxel intravenously every three weeks in our center. This regimen was recommended by the NCCN guidelines, combined with oral prednisone (5mg/twice a day) and ADT (Zoladex 3.6mg/once every 28 days). The experimental protocols were approved by the institutional ethics committee of Shanghai Ren Ji Hospital affiliated with Shanghai Jiao Tong University (Approval No. Renji [2013]126). All patients were informed of the study in detail and their written informed consents had been obtained.
The evaluation of docetaxel chemotherapy response in patients with mCRPC was based on the Prostate Cancer Working Group 3 (PCWG3) standard.18 Prostate-specific antigen (PSA) response (sensitive to docetaxel) was defined at least after 12 weeks (four chemotherapy cycles), the plasma PSA level should decrease to below 50% of the baseline level and maintained above four weeks. Otherwise, the patient was judged to be PSA failure (insensitive to docetaxel).
To monitor the response and toxic side effects of docetaxel in mCRPC patients, all enrolled patients were required to review PSA, CBC, liver and kidney function, electrolytes, blood lipids, and blood glucose after every chemotherapy cycle. A Bone scan or CT or MRI should be performed every 12 weeks in order to assess the imaging response to docetaxel-based chemotherapy. We also arranged professionals who had been well trained to follow up on the changes of clinical symptoms and life quality of patients regularly.
2.2. Clinical and pathological features and blood biochemical parameters
This study analyzed seven clinical-pathological parameters of all patients, including (age at chemotherapy, bone pain, Gleason score, castration sensitive duration under ADT, pre-chemotherapy PSA, metastases, and EOD score) and four blood biochemical parameters, Including (hemoglobin, alkaline phosphatase, lactate dehydrogenase, and ratio of C-reactive protein to albumin). All relevant biochemical and imaging findings and other clinical- pathological data could be found in the electronic medical record information system of our hospital.
2.3. Collection of serum samples and Raman spectrometer detection
The time of collection of serum samples at baseline (qq0) was within three days before chemotherapy. The time of collection of serum samples after qq1 was within one week after the first chemotherapy (q1q1). 5ml of venous blood was collected after 12h of overnight fasting. The samples were centrifuged at 3000rpm for 5min, and serum was collected afterwards and frozen at −80∘−80∘C until Raman spectrometer detection.
Silver nanoparticles (AgNPs) were synthesized by using the sodium citrate reduction method.19 The AgNPs were observed using a transmission electron microscope (Fig. 1). After preprocessed serum samples were thawed, 20μμl of serum was mixed with 40μμl of AgNPs solution and allowed to stand at room temperature for 10min. 10μμl of the mixture was pipetted onto a silicon wafer and placed on an automated platform of an inverted microscope attached to a Raman spectrometer to prepare for detecting the Raman spectral signal of the specimen. In this study, a 633nm He–Ne laser with a laser power of about 3.5mW was focused on the sample surface. A Raman microscope (The Renishaw, LONDON, UK) was used for the collection of SERS spectra. The Leica DM2500 microscope camera (Leica Microsystem, Weltzlar, Germany) was used for signal acquisition. Each acquisition time was set at 10s. Raman signal of each sample was collected at three random different points.
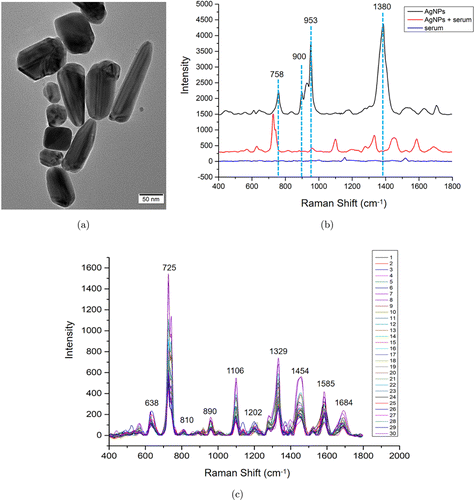
Fig. 1. (a) Transmission electron microscope of Ag nanoparticals. (b) Raman spectra of Ag nanoparticals, mixture of Ag nanoparticals and serum, and serum. (c) The individual SERS spectra of serum from 10 CaP patients.
2.4. Raman spectroscopy graphic analysis and data processing
The Raman spectra data were collected by WiRE 3.2 software. The Raman spectra was set in the range of 400–1800cm−1−1 (Fingerprint region). The original spectra collected by the spectrometer were pretreated by OriginPro 8 software, including background subtraction and spectral area homogeneity. Four mean and subtracted spectra have been eventually generated, which were as follows:
| • | Normalized mean SERS spectra of PSA response group at qq0; | ||||
| • | Normalized mean SERS spectra of PSA response group after qq1; | ||||
| • | Normalized mean SERS spectra of PSA failure group at qq0; | ||||
| • | Normalized mean SERS spectra of PSA failure group after qq1. | ||||
We compared the alters and differences of relevant Raman peaks between (1) and (2), (3) and (4), (1) and (3), (2) and (4), respectively. In order to analyze the molecules represented by the Raman peaks of SERS spectra, this study referred to the relevant Raman library and previous literature reports. Clinical variables and spectra data were compared by using Mann-Whitney UU test, independent-sample tt test, and chi-squared test appropriately. PSA response of patients with mCRPC after chemotherapy was described by waterfall chart; principal component analysis and linear discriminant analysis (PCA-LDA) were established to analyze the spectra data and create a predictive model. The performance of the predictive model was trained and tested using the five-cross-validation method and leave-one-out cross-validation method. All statistical analyses were performed using the RR-statistical package (R Foundation for Statistical Computing, Vienna, Austria), with P<0.05P<0.05 as statistically significant.
3. Results
This study included 39mCRPC patients who received docetaxel chemotherapy. Seven of them discontinued chemotherapy due to serious side effects during treatment (two patients had severe liver damage and two patients had serious infections, the remaining three patients developed upper gastrointestinal bleeding). According to the Prostate Cancer Working Group 3 (PCWG3) standard, the response of docetaxel could not be evaluated without completing four times of chemotherapy, so these seven patients were excluded. The remaining 32 patients completed at least four times of chemotherapy. The detailed clinicopathological features and plasma biochemical parameters of two groups at baseline are shown in Table 1.
According to PCWG3 criteria, all patients were divided into PSA response group (n=17n=17) and PSA failure group (n=15n=15). There were no significant differences in the relevant clinicopathological characteristics or blood biochemical indexes at baseline between two groups (p>0.05p>0.05). The median ADT PSA progression free time was 27 months in PSA response group, and 16 months in PSA failure group. Although the above comparison was not statistically significant (P=0.12P=0.12), among those patients with castration sensitive duration under ADT less than 12 months, 83% (5/6) patients got initial resistance of docetaxel. The result of the maximum PSA decline of all patients with mCRPC after docetaxel chemotherapy is shown in Fig. 2. There were 16 (53%) patients with PSA decline >30%>30%, and 15(50%) patients with PSA decline >50%>50%.
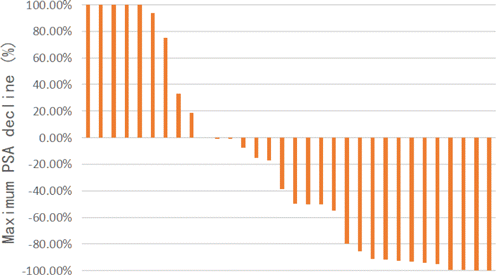
Fig. 2. Waterfall plot of the response of PSA in patients with mCRPC after docetaxel chemotherapy.
| PSA response (n=17n=17) | PSA failure (n=15n=15) | PP value | |
|---|---|---|---|
| Median age, years | 69.8 | 67.7 | 0.34 |
| Significant pain, nn (%) | 8 (47%) | 6 (40%) | |
| Gleason score, nn (%) | |||
| Unknown | 1 (6%) | 3 (20%) | |
| ≤7≤7 | 6 (35%) | 5 (33%) | |
| ≥8≥8 | 10 (59%) | 7 (67%) | 0.88 |
| Median castration sensitive duration under ADT, months | 27 | 16 | 0.12 |
| Median PSA at docetaxel initation, ng/ml | 187.9 | 234.4 | 0.33 |
| Metastatic site, nn (%) | |||
| Only bone metastases | 17 | 15 | |
| Bone and visceral metastases | 0 | 0 | |
| EOD performance status, nn (%) | |||
| ≤1≤1 | 6 (35%) | 3 (20%) | |
| 2 | 2 (12%) | 0 | |
| 3 | 9 (53%) | 12 (80%) | 0.63 |
| Median serum markers at the start of docetaxel therapy | |||
| Hemoglobin, g/dL | 132 | 124 | 0.10 |
| ALP, IU/L | 98 | 116 | 0.87 |
| LDH, IU/L | 202 | 187 | 0.20 |
| CRP-to-Albumin ration | 0.04 | 0.16 | 0.05 |
Figure 1 demonstrates the performance of SERS measurement. Figure 1(a) shows the TEM image of AgNPs, which displays that the size of AgNPs varies from 40nm to 120nm. Figure 1(b) shows the Raman spectra of AgNPs that mainly contain four peaks, including 758, 900, 953, and 1380cm−1−1. Compared with the normal Raman spectra of serum, the SERS spectra of serum showed abundant and distinct Raman peaks, however, those SERS spectra of serum contain none of the spectra peaks from AgNps. The individual raw SERS spectra of serum from 10 CaP patients are shown in Fig. 1(c), the Raman peaks are stable and reproducible among different serum samples; at the same time, the intensities of Raman spectra are distinct, indicating that SERS analysis might reveal the unique molecular information of serum in different patients.
Totally, we measured 17 serum SERS spectra from PSA response group at qq0; 17 serum SERS spectra from the PSA response group after qq1; 15 serum SERS spectra from the PSA failure group at qq0; 15 serum SERS spectra from the PSA failure group after qq1. The normalized mean serum SERS spectra of two groups at qq0 and after qq1 were shown in Figs. 3 and 4, respectively. Upon comparing the mean SERS spectra of the PSA response groups at qq0 and after qq1, some discernible differences can be found at peaks 638(p<0.05p<0.05), 725(p<0.05p<0.05), 810, 890, 1106, 1136(p<0.05p<0.05), 1202(p<0.05p<0.05), 1329, 1454, 1585, and 1684cm−1−1(p<0.05p<0.05). In order to study the molecular information in serum provided by Raman spectroscopy, we analyzed the substances revealed through serum SERS by consulting Raman spectroscopy database and previous literature reports (Table 2).
| Raman shift cm−1−1 | Vibrational mode/Assignment |
|---|---|
| 638 | ττ(C–S)/Tyrosine |
| 725 | bυυ(C–H)/Adenine |
| 810 | s(C–C–O)/L-serine |
| 890 | b(C–O–H)/D-Amino galactose |
| 1106 | Amide III |
| 1136 | ss(C–H)/D-mannose |
| 1202 | rv/Tryptophan, phenylalanine |
| 1329 | CH3CH2/Purine bases |
| 1454 | bυυ(CH3) and δδ (CH2)/Elastin, collagen, and phospholipids |
| 1585 | bυυ(C=C)/Acetoacetate |
| 1684 | Amide I |
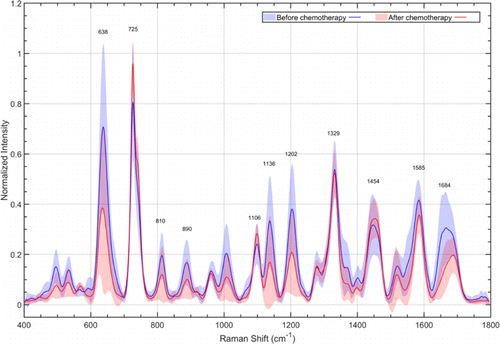
Fig. 3. Normalized mean SERS spectra of PSA response group at qq0 (blue) and after qq1 (red). The solid lines represent the mean spectra, while the gray zones represent the standard deviation.
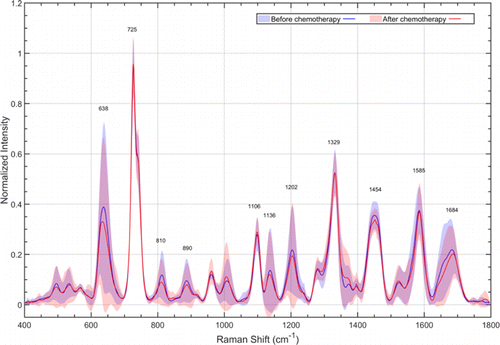
Fig. 4. Normalized mean SERS spectra of PSA failure group at qq0 (blue) and after qq1 (red). The solid lines represent the mean spectra, while the gray zones represent the standard deviation.
In contrast to the results in PSA response group, the characteristic Raman peaks of PSA failure group at qq0 and after qq1 almost completely coincided with each other, and the relative peak intensity did not change significantly (p>0.05p>0.05).
The normalized mean serum SERS spectra of PSA response group (red) and PSA failure group (blue) at qq0 were shown in Fig. 5. There were discernible differences in SERS peaks of 638cm−1−1 (tyrosine) (p<0.05p<0.05), 725cm−1−1, 810cm−1−1 (Adenine) (p<0.05p<0.05), 890cm−1−1 (D-Amino galactose) (p<0.05p<0.05), 1106cm−1−1, 1136cm−1−1 (D-mannose) (p<0.01p<0.01), 1202cm−1−1 (Tryptophan, phenylalanine) (p<0.05p<0.05), 1329, 1454, 1585 and 1684 cm−1−1. In contrast, while analyzing the normalized mean SERS spectra of PSA response group (red) and PSA failure group (blue) after qq1, which were shown in Fig. 6, we could directly observe that the positions and intensity of the characteristic peaks of the Raman spectra of two groups were basically the same.
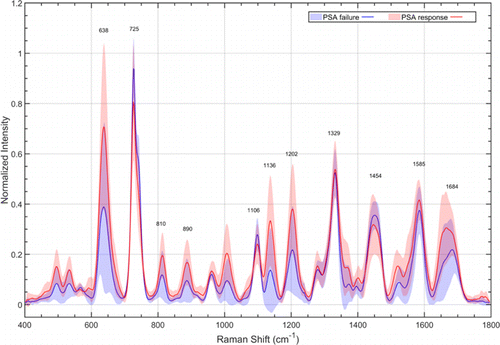
Fig. 5. Normalized mean SERS spectra of PSA response group (red) and PSA failure group (blue) at qq0. The solid lines represent the mean spectra, while the gray zones represent the standard deviation.
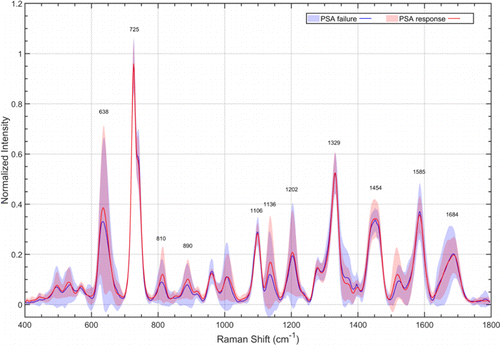
Fig. 6. Normalized mean SERS spectra of PSA response group (red) and PSA failure group (blue) after qq1. The solid lines represent the mean spectra, while the gray zones represent the standard deviation.
The foregoing only compared the relative intensity of Raman peaks of serum before chemotherapy between PSA response and PSA failure group, which might be feasible to some extent. Among those Raman peaks, the 1,136cm−1−1 showed the optimal ability for discriminating patients of the two groups, and the detailed information were shown in Fig. 7. PCA and linear discrimination analysis (LDA) were further applied to discriminate two groups. PCA is a multivariate technique that can capture several important principal component variables in complex data. LDA is a model for discrimination between groups by maximizing variance and minimizing variance within groups. We trained the PCA–LDA model with accumulating feature counts and validated it using the five-cross-validation method. The results are shown in Fig. 8 and we concluded that the training accuracy of the PCA–LDA model is increasing with the accumulation of feature counts, which indicated that the model is over-fit. However, according to the results of five-cross-validation, the PCA–LDA model reached the optimal testing accuracy when the primary four feature counts were included, revealing the accuracy of 0.73 and AUC of 0.83.
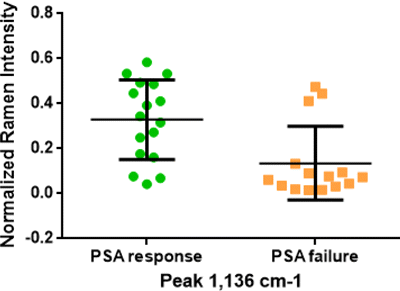
Fig. 7. Box plots of relative intensity of Raman peak 1136cm−1−1 in two groups.
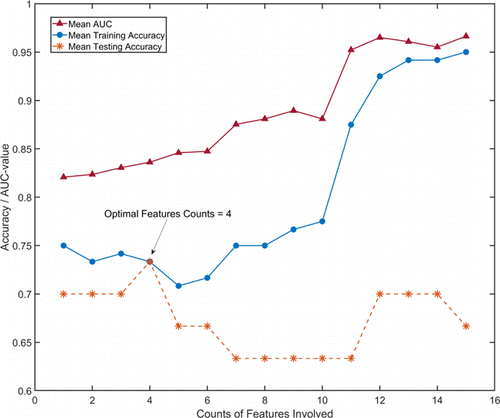
Fig. 8. The performance of PCA and LDA model when different counts of features are involved. five-cross-validation method is used to train and test the model. The arrow shows the best testing accuracy when the primary four features counts are included.
In order to verify effectiveness of the discrimination prediction models, we used the LOOCV method to verify the relative intensity of peak 1136cm−1−1 and the PCA–LDA discrimination model, respectively. Verification results of the relative intensity of peak 1136cm−1−1 discrimination model showed that 12 of 17 in PSA response group and 12 of 15 in PSA failure group were correctly classified. As shown in Table 3, the sensitivity, specificity, and accuracy to predict response to docetaxel chemotherapy in patients with mCRPC were 70.6%, 80%, and 75%, respectively. The verification results of the PCA–LDA discrimination model showed that 12 of 17 in PSA response group and 11 of 15 in PSA failure group were correctly classified, and the sensitivity, specificity and accuracy of which were 69%, 78%, and 73%, respectively.
| Raman peak 1136cm−1−1 | PCA–LDA model | |
|---|---|---|
| Sensitivity (%) | 71 | 69 |
| Specificity (%) | 80 | 78 |
| Accuracy (%) | 75 | 73 |
The above results indicated that both Raman peak 1136cm−1−1 and PCA–LDA model based on the data of serum SERS before chemotherapy had a pretty good ability to predict sensitivity to docetaxel in mCRPC.
4. Discussion
The data of TAX327 phase III clinical trial suggested that some clinical-pathological features and blood biochemical index were significantly associated with the prognosis of mCRPC who received docetaxel chemotherapy, including physical condition, liver metastasis, clinically significant pain, PSA doubling time, PSA baseline, tumor stage, the content of serum alkaline phosphatase, and hemoglobin.4 To investigate whether some clinical factors of mCRPC could reflect sensitivity to docetaxel of mCRPC, this study analyzed and compared the seven clinicopathological indexes and four blood biochemical indexes. The above results showed that the clinical indexes related to the invasion degree of CaP and general basic conditions had certain predictive effect on the prognosis of mCRPC after chemotherapy instead of response to docetaxel. It was worth noting that the median castration sensitive duration under ADT in PSA response group of chemotherapy was 27 months, which was greater than that of 16 months in PSA failure group, but the difference between two groups was not statistically significant (p>0.05p>0.05). The result was quite in concordance to data from the phase III clinical trials of VENICE and TAX327, which showed that castration sensitive duration was related to the prognosis of mCRPC patients after docetaxel chemotherapy, but not significantly related to the response of docetaxel.24 50% of mCRPC patients in our cohort had a PSA response ≥≥50% after chemotherapy, which was also consistent with the results of the phase III clinical trials, TAX327 and SWOG9916.
Due to the heterogeneity of tumors and the complexity of the mechanism of docetaxel resistance, there were limited studies on biomarkers that could successfully predict docetaxel sensitivity. Overexpressed ERG could bind to soluble tubulin and affect the activity of microtubules, resulting in decreased sensitivity of docetaxel.25 Song et al., performed immunohistochemical staining on the samples of 72 mCRPC patients who had received docetaxel chemotherapy and found that patients with positive ERG expression had lower PSA response rate (15.4% versus 62.1%, p=0.004p=0.004) compared with those with negative ERG expression.26 TMPRSS2-ERG fusion gene could promote the transformation of prostate epithelial cells into stroma, leading to docetaxel resistance ultimately.27 Oscar Rei et al., found that TMPRSS2-ERG fusion gene was a biomarker that could effectively predict the sensitivity of mCRPC patients after docetaxel, and the PSA response rate of TMPRSS2-ERG positive mCRPC patients after docetaxel was significantly lower (12.5% versus 68.3%, p=0.005p=0.005).28 Although the above studies showed that ERG or TMPRSS2-ERG fusion gene had certain potential to predict sensitivity to docetaxel in mCRPC, their cohort sizes were generally small and were not verified in the external population. So far, there has not been a widely accepted method to predict sensitivity to docetaxel chemotherapy in mCRPC patients, and a new method is urgently needed to solve this problem.
Serum SERS detection technology, as a noninvasive molecular detection method, has many advantages such as convenient to prepare samples, fast, and high specificity. All molecular information displayed by serum SERS can be further explained and analyzed using statistical, chemical, and morphological methods.29 In order to explore the ability of serum SERS to predict docetaxel sensitivity in mCRPC, a total of 64 matched serum Raman spectra from 32 mCRPC patients at q0q0 and after q1q1 were included in this study. Compared with SERS spectra of PSA response group at q0q0, the intensities of Raman peaks of the PSA response group at q1q1 have significantly declined, including 638cm−1−1 (tyrosine), 725cm−1−1 (adenine), 1136cm−1−1 (D-mannose), 1202cm−1−1 (tryptophan, phenylalanine), and 1684cm−1−1 (amide I), p<0.05p<0.05. These changes indicated that the positive effect of docetaxel on tumor burden decreased the concentration of molecules (glycogen, nucleic acids, and amino acids) which were related to the tumor metabolism in serum. Monitoring the changes of these characteristic Raman peaks continuously in the course of chemotherapy could help us to judge the therapeutic effect of docetaxel in mCRPC and the time of drug resistance. In contrast, for the PSA failure group, the intensities of Raman peaks barely changed after q1q1. It could be concluded that invasiveness of CaP in the PSA failure group did not decrease after chemotherapy and initial docetaxel resistance occurred.
By comparing serum SERS at q0q0 between PSA response group and PSA failure group, it was found that SERS in two groups revealed significant differences, mainly at 638cm−1−1 (tyrosine), 890cm−1−1 (D-galactosamine), 1202cm−1−1 (tryptophan, phenylalanine), 1136cm−1−1 (D-mannose), and 1684cm−1−1 (amide I), p<0.05p<0.05. Among them, 1136cm−1−1 was the peak with the largest difference between two groups, p=0.003p=0.003; As mentioned above, the intensity of peak 1136cm−1−1 significantly has declined after q1q1 in PSA response group, but it changed little after q1q1 in PSA failure group. All these results indicated that the intensity of peak 1136cm−1−1 could well predict the response to docetaxel in mCRPC patients. After consulting relevant literatures, it was found that the chemical bonds and substances attributed to peak 1136cm−1−1 were ββ (C–H), D-mannose. Gonzalez et al., found that mannose could inhibit the growth of a variety of tumors by interfering with the glucose metabolism of tumor cells. After being fed with mannose supplements, tumors of pancreatic, skin and lung cancers in mice were effectively suppressed. Mannose could also significantly enhance the therapeutic effect of chemotherapy drugs such as cisplatin and adriamycin, moreover the combination of mannose and the above chemotherapy drugs could achieve better tumor inhibition effect.30 Although this study did not indicate whether mannose can sensitize docetaxel, combined with the results of our study, mannose may also have the ability to enhance the sensitivity of docetaxel to chemotherapy. Future in vitro and in vivo studies are needed to investigate the role of mannose in docetaxel chemotherapy.
This study conducted the same research on spectra of serum after the first chemotherapy. Contrary to the above result, no significant difference in the intensity of Raman peaks between two groups was observed. It demonstrated that serum SERS after the first chemotherapy did not have the value of predicting the sensitivity of docetaxel in mCRPC, and also the difference in the concentration of molecules represented by the Raman peaks between two groups after q1q1 was much smaller than that at q0q0. It was hard to explain why the Raman spectra could not detect the difference in the content of related molecules between the two groups after the first chemotherapy. The possible hypothesis was that after the first chemotherapy, the tumor load of docetaxel-sensitive mCRPC patients greatly relieved, resulting in a significant decrease in the serum concentration of molecules related to docetaxel sensitivity. The result may be consistent with the observation in clinical practice that patients with mCRPC will gradually develop resistance to docetaxel from initial sensitivity. Therefore, in order to solve the above problems and verify the hypothese, it is indispensable to continuously monitor the changes of serum Raman spectra of mCRPC patients during all chemotherapy cycles (q0q0, q1q1, q2q2, q3q3, and so on) and verify relevant conclusions through subsequent cell lines and animal experiments.
Individual quantitative analysis of Raman peak can only provide limited information for discrimination. In order to make full use of whole SERS spectral data for predicting sensitivity to docetaxel chemotherapy in mCRPC, PCA–LDA was applied to further analyze the spectral data. According to the results of five-cross-validation, the PCA–LDA model reached the optimal testing accuracy when the primary four feature counts were included, revealing accuracy of 0.73 and AUC of 0.83. To get the actual effectiveness of the prediction model, we used LOOCV method to verify the PCA–LDA model, and determined its prediction sensitivity, specificity, and accuracy to be 69%, 78%, and 73%, respectively. The peak intensity of 1136cm−1−1 based on the serum SERS spectra before chemotherapy was determined with a sensitivity of 71%, specificity of 80% and accuracy of 75%. The above results indicated that the PCA–LDA model and the Raman peak 1136cm−1−1 based on the serum SERS spectra before chemotherapy were valuable to predict sensitivity to docetaxel chemotherapy in patients with mCRPC.
Several limitations for our research should be stated. First, our study is a single-center research with a small sample size, so bias and compromised generalizability are inevitable. It is necessary to further verify the effectiveness of the above prediction model in an external population with a larger sample size. Second, the substances changes reflected by SERS are on the basis of tentative assignments in published documents and Raman spectral database, which should be further confirmed by other methods in future studies. With the advantages of convenience, noninvasiveness, and high specificity, it is believed that in the near future, serum SERS can provide important references for clinicians in various clinical fields, such as early diagnosis of cancer, prognosis prediction of radical surgery, and prediction of chemotherapy sensitivity.
Conflicts of Interest
The authors declare that there is no conflict of interests relevant to this paper.
Acknowledgments
The study was supported by Clinical Research Plan of SHDC (No. SHDC2020CR3014A) and National Natural Science Foundation of China (Grant No. 82003148). Jianian Hu and Xiaoguang Shao contributed equally to this work.


