Green HPTLC-method to estimate trans-Cinnamaldehyde in Ayurvedic formulation Sitopaladi Churna
Abstract
Traditional medicine is a preferred home remedy for common cold, cough, allergic conditions, etc. in South East Asia. Ayurveda, originated in India, has a long history of treating upper respiratory disorders. Sitopaladi churn (powder) is one of the most sold “over-the-counter” herbal medicine for upper respiratory ailments cure. Quality control and validation is a hindrance for Ayurvedic product development. This study was conducted to estimate and validate for quantitative study of trans-Cinnamaldehyde in Ayurvedic medicine Sitopaladi Churna (SPC) through high-performance thin-layer chromatography (HPTLC) method. The quantitative study was performed at the wavelength 294nm and validated as per ICH guidelines. The content of trans-Cinnamaldehyde in SPC was found to be 0.093%. Green HPTLC method is simple and sensitive for standardization and is validated for trans-Cinnamaldehyde in Sitopaladi powder. This method would add a different approach for quality control and assay of SPC.
Introduction
High-performance thin-layer chromatography (HPTLC) method has been a promising method because efficient separation, identification, and assay for many phytochemicals are present in Ayurveda medicines. The reducing silica particles enhance faster analysis, better resolution, sharper peaks, sensitivity, and cost-effective method to facilitate improving the separation and quantification of HPTLC. The few micrograms sample are enough to observe and quantify a target to analyze on HPTLC. A major benefit of HPTLC method is the application of multiple samples and sequential manner, separated, and qualitative and quantitative standardization in a single run therefore this method is easy for polyherbal Ayurvedic medicines.1,2
Various chromatography techniques are applied for the separation of trans-Cinnamaldehyde separation like HPTLC and HPLC. In HPTLC method, in solvent system cyclohexane, ethyl acetate (90:10, v: v), offered good separation and retardation factor of Cinnamaldehyde at Rf=0.27±0.01.3 Another method in the form of the reversed-phase HPLC analysis was carried out using an Intersil ODS-3V-C18 (150mm×4.6mm, 5μm) column and a mobile phase comprising methanol-acetonitrile-water in the volume ratio of 35:20:45, delivered at a flow rate of 1.0cm3/min. The detection and quantitation of both the compounds were carried out at 221nm. The method uses gradient mode with retention time of cinnamaldehyde as 10.2min while the present developed method uses isocratic mode with retention time of 5.95min for cinnamaldeyhde.4
Ayurveda has gained worldwide acceptability for curative and preventive purposes for numerous chronic diseases like arthritis, diabetes, gastrointestinal disorders, immune disorders, and liver diseases. Ayurveda formulations like Hingavastaka churna, Sitopaladi churna (SPC), Trikatu churna, Sringyadi churna, and Avipattikara churna are most frequently available in the market.5,6,7
SPC is a Ayurvedic formulation prescribed for allergy, cold, cough, pneumonia bronchitis, tuberculosis, intercostal neuralgia, burning sensation in extremities, viral respiratory infection, digestive impairment, and in pharyngeal and chest congestion.5 Trans-Cinnamaldehyde, naturally occurring flavonoids (trans – 3 phenyl-2 propenal; C6H5CH=CHCHO) (Fig. 1) is the active principle in SPC. Modern research confirmed that trans-Cinnamaldehyde is helpful to treat tooth decay, bad breath, and enhances oral health and antibacterial and antifungal property.8 The investigation needs standardization parameters. Therefore, this study aimed to establish fingerprint profile and quantification of trans-Cinnamaldehyde in SPC to improve quality control tool.9
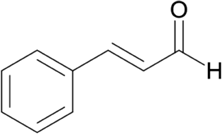
Figure 1. Chemical structure of trans-Cinnamaldehyde.
Experimental Design
Chemicals
Trans-Cinnamaldehyde was bought from Sigma-Aldrich, USA. Absolute Ethanol was from Chong Yu Hi-Tech Chemicals–China, Toluene from Loba Chem. Pvt. Ltd, and Ethyl acetate from Merck Specialty Chem. Ltd., Formic acid from Thermo-fisher Scientific Ltd. All chemicals used were of analytical grade.
Collection of plant materials and identification
Sitopaladi Churna is poly-herbal formulation that contains Mishri (Saccharum Officinarum), Vanshalochan (Bambusa Arundinacea), Pippali (Piper Longum), Ela (Elettaria Cardamomum), and Dalchini (Cinnamonum Zeylanicum) as ingredients, as shown in Table 1. The SPC was prepared in laboratory in the mentioned ratio and the raw herbs were procured from the local markets of Raipur, Chhattisgarh, India, and authenticated at Drugs Testing Laboratory Avam Anusandhan Kendra, Raipur, Chhattisgarh.
| S. No | Sanskrit name | Botanical name | Part used | Quantity |
|---|---|---|---|---|
| 1. | Twak | Cinnamonum Zeylanicum | Dried Stem Bark | 1 Parts |
| 2. | Ela | Elettaria Cardamomum | Dried Seed | 2 parts |
| 3. | Pipali | Piper Longum | Dried Fruit | 4 parts |
| 4. | Vansalochan | Bambusa Arundinacea | White part (siliceous Concretion) | 8 parts |
| 5. | Sitopala | Saccharum Officinarum | Sugar Candy | 16 parts |
Preparation of Sitopaladi Churna
Accurately weighted and mixed Dalchini (Cinnamonum Zeylanicum) (1g), Elachi (Elettaria Cardamomum) (2g), Pipali (Piper Longum) (4g), Vansalochan (Bambusa Arundinacea) (8g), and another parts Sitopala (Saccharum Officinarum) (16g) were pulverized and passed through Sieve no 60. The above cited samples were mixed properly and pulverized and filtrated with sieve no 80. The samples were stored in a proper container. The quality standardization was evaluated by the physicochemical parameters of the prepared SPC as per the guideline in Ayurvedic Pharmacopoieas of India. SPC was prepared as per Sharangdhar Samhita Ayurvedic book mention in schedule I, D&C Act 1940 (Fig. 8).
Preparation of standard solution
Accurately weighed 100mg Trans-Cinnamaldehyde was dissolved in 100ml of ethanol solvent; 1mg/mL of stock solution was prepared. A standard operational solution (0.05mg/mL or 50μg/mL) was prepared of Trans-Cinnamaldehyde by diluting the stock solution with ethanol solvent.
Preparation of sample solution
The SPC (5g) was accurately weighed and dissolved in 50ml ethanol solvent in a Stoppard conical flask with 20min sonication in bath ultra-sonicator. Before analysis, the solution was filtered with 0.45μm syringe filter. The sample solution (15μL/band) was applied. The sample solution was prepared in a triplicate (n=3).
HPTLC method development
HPTLC partition was performed on a silica gel-G HPTLC plate F254 (20cm×10cm with 0.2mm thickness; Merck, Darmstadt, Germany). Tests and Trans-Cinnamaldehyde standard arrangement were connected at bandwidth of 8mm and removed from the lower edge at 8mm by a Linomat 5 programmed test spotter (CAMAG, Switzerland). A steady application rate of 100nL/s was utilized. The plate was created in a CAMAG twin trough glass chamber, pre-saturated for 20min by creating a dissolvable solution comprising Toluene, Ethyl acetate, and Formic acid (5:2:0.5%v/v/v). The plate was topped up to 70mm of solution. Densitometric checking was performed at 294nm by utilizing TLC scanner 4 (CAMAG, Switzerland). The opening measurement was 6.00mm×0.45mm with a checking speed of 20mm/s. Densitograms were analyzed by Vision CATs computer program.
Validation method
The proposed strategy was approved in terms of Linearity, LOD and LOQ, Reproducibility, Precision and Recovery concurring to the International Conference on Harmonization rule (ICH 1996/2005).10
Linearity
Trans-Cinnamaldehyde (50 μg/mL), was applied at 0.5−3.5μL, compared to the concentration of Trans-Cinnamaldehyde at 25−175 ng/band, independently connected on the HPTLC plate. The calibration curves were received by plotting between the peak areas against the concentration of standard Trans-Cinnamaldehyde.
Precision
LOD and LOQ were decided based on the standard deviation of y-intercepts of regression curve (SD) and the slope of the calibration curve (S) of the test within the range of LOD, LOQ utilizing the the following equation :
Reproducibility
For the consideration of repeatability concentrations of a standard arrangement, 100ng/band was connected on the HPTLC plate (n=3). Repeatability was decided by investigation of Trans-Cinnamaldehyde at three distinctive time intervals inside one day, whereas the middle of the road exactness was decided on three sequential days by utilizing the proposed method. The precision was communicated as a rate of relative standard deviation (% RSD).
Recovery
The accuracy of the strategy was affirmed by the estimation of the recovery. The SPC was utilized for the recovery test. Three diverse concentrations of Trans-Cinnamaldehyde (around 80%, 100%, and 120%) were included in the SPC. Spiked tests were arranged in triplicate.
Specificity
The specificity described that the standard Trans-Cinnamaldehyde present in the SPC and other constituents applied to determination should not interfere with any solvent, sample and mobile phase.
Results and Discussion
Determining quality standardization is a major aspect of polyherbal medicine. The physicochemical tests were allowed inside restrain values and assured for their qualities (Table 2).
| S. No. | Physicochemical parameter for SPC | Observed value | Reference value (As per Ayurvedic Pharmacopeia)12 |
|---|---|---|---|
| 1 | Particle fitness | Fine Powder | Fine Powder |
| 2 | Loss of drying at 105∘C (%) | 4.60 | NMT-7 |
| 3 | Total Ash (%) | 15.50 | NMT-16 |
| 4 | Acid Insoluble Ash (%) | 6.50 | NMT-15 |
| 5 | Alcohol soluble extractive (%) | 23.90 | NLT-6 |
| 6 | Water soluble extractive (%) | 56.20 | NLT-55 |
HPTLC method is appropriate as an alternative strategy for the scheduled examination and quality control of herbal pharmaceutical due to its effortlessness, accuracy, and suitability for high-throughput screening. So, it may be a favored analytical device for fingerprint examination and quantification of marker compounds Trans-Cinnamldehyde in SPC. The optimization of the versatile stage was done by utilizing different solvents. The optimized mobile phase consisting of Toluene, Ethyl acetate, and Formic acid (5:2:0.5%v/v/v) showed an acceptable resolution of standard Trans-Cinnamaldehyde and in the sample SPC at 294nm. SPC sample, Standard Trans-Cinnamaldehyde, and Mixed Sample (SPC sample, Trans-Cinnamaldehyde) are applied on the HPTLC plate. The bands were shown in both SPC sample and Trans-Cinnamaldehyde standard at Rf 0.635±0.035 (Fig. 2) and fingerprint analysis was also shown (Fig. 3). The specificity of Trans-Cinnamaldehyde in SPC sample was confirmed with an overlay UV spectra amid the reference standard Trans-Cinnamaldehyde and SPC sample (Fig. 4). From the UV spectra overlay, the wavelength maxima 294±1nm showed no obstructions of other compounds; in this manner, the maximum retention of Trans-Cinnamaldehyde at 294±1nm was chosen for the investigation. The purity is calculated by measuring three spectra inside one top, one low, and middle range, and comparing these measurably. Peak purity test appeared at a high degree of relationship between the spectra that were filtered at peak begin, peak pinnacle, and peak end positions of Trans-Cinnamaldehyde. The HPTLC optimized method is used to create fingerprinting and assay. The developed method was useful for the analysis of active molecule trans-Cinnamaldehyde content in SPC samples. According to the HPTLC method analysis, Trans-Cinnamaldehyde content in SPC sample was found to be 0.093±0.021%.
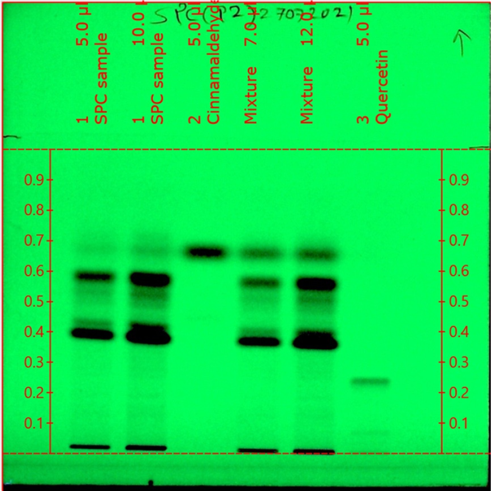
Figure 2. Fingerprinting of SPC with trans-Cinnamaldehyde.
Figure 3. Chromatograph of SPC.
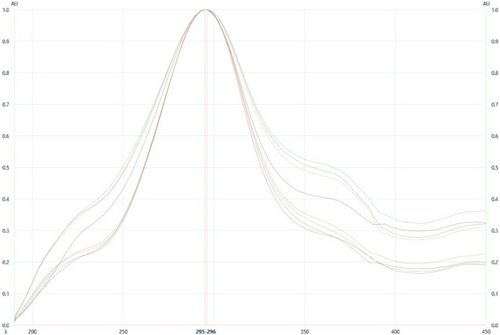
Figure 4. UV spectra overlay of trans-Cinnamaldehyde (Rf=(0.635±0.035)).
The HPTLC method was validated with Trans-Cinnamanldehyde in a SPC by applying the parameter, linearity, LOD, and LOQ. Precision and Recovery were examined through this (ICH 1996/2005).10 The accepted validation parameters were shown (Table 3). The calibration curves of Trans-Cinnamaldehyde were found to be linear over the range of 25–175ng/band (Fig. 5). The Coefficient of variance (CV) was lesser than 2.4% demonstrating great linearity of the strategy as well as reproducibility and intermediate precision were moreover less than 2.4% (Fig. 6). The LOD and LOQ were found to be 0.336 and 1.018ng/band individually. The LOD and LOQ were the lowest concentrations of Trans-Cinnamaldehyde in a test sample which can be recognized and evaluated under the experimental conditions individually. The recovery of Trans-Cinnamaldehyde in SPC was within the range of 91.35±1.35% demonstrating a high accuracy of the method (Fig. 7).
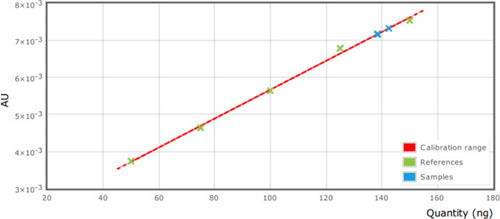
Figure 5. The calibration curves of trans-Cinnamaldehyde at 294nm.
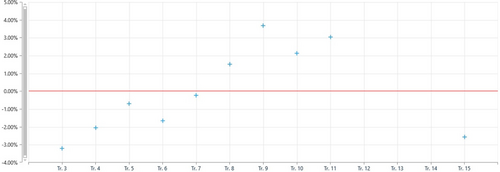
Figure 6. Reproducibility studied of trans-Cinnamaldehyde at 294nm.
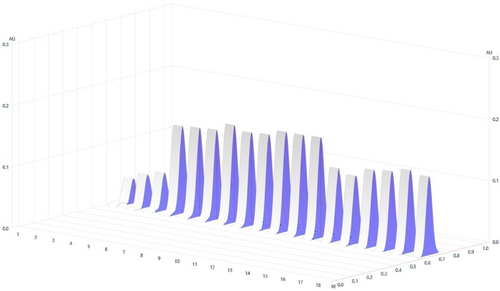
Figure 7. Recovery studied of trans-Cinnamaldehyde at 294nm.
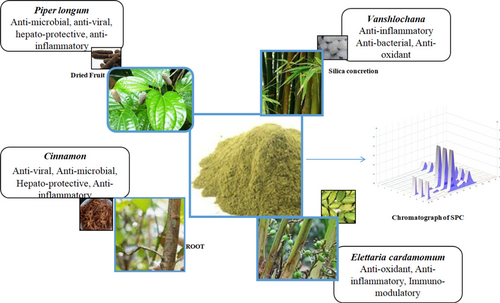
Figure 8. The Sitopaladi Churna and its ingredients with uses.
| Parameters | Trans-Cinnamaldehyde |
|---|---|
| Range of linearity | 25–75ng band−1 |
| Regression equation | Y=3.896×10−8X+1.774×10−3 |
| Correlation coefficient (R) | 99.8% |
| Reproducibility (%) | 2.4% |
| Recovery (%) | 91.35±1.35% |
| Limit of detection (ng) | 0.336ng |
| Limit of quantitative (ng) | 1.018ng |
| Coefficient of variation (CV) % | 1.39% |
| Content in SPC (%) | 0.093±0.021% |
| Rf | 0.635±0.035 |
| Solvent | Ethanol |
| Mobile Phase | Toluene: Ethyl acetate: Formic acid (5:2:0.5%v/v/v) |
| Wavelength maxima | 294nm |
Statically Study of Data
The information was represented as a mean±standard deviation. The expressive measurements were conducted at whatever point it was appropriate.
Conclusion
The straightforward and delicate HPTLC method was effectively created and approved for the validation of SPC. The proposed HPTLC method appeared to have satisfactory approval parameters. This method offered a few points of interest counting effortlessness, fast, numerous tests, less solvent utilized, less time of investigation, and less cost per investigation when compared to the HPLC method. Subsequently, this approved HPTLC method can be utilized as an alternative method for quantitative investigation of SPC and other herbal preparations containing Trans-Cinnamaldehyde as marker Compound.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors express their gratitude to the Director of Ayush (Chhattisgarh) for laboratory facilities and National Ayush Mission (NAM) Grant-2016-17 for financial support provided by HPTLC.


