Value of Meiotic Spindle Imaging on Fertilization and Embryo Development Following Human Oocyte Piezo-ICSI
Abstract
Background: The objective of this study was to investigate accurately the effect of the meiotic spindle imaging of human oocytes on their fertilization and embryo development by using the Piezo-ICSI.
Methods: We retrospectively assessed 529 oocytes with the first polar body retrieved from 124 infertile couples (147 cycles) who attended our clinic between May 2016 and December 2018. Of these, 489 oocytes (92.4%) with visible meiotic spindle comprised the Spindle (+) group and 40 oocytes (7.6%) not observed meiotic spindle comprised the Spindle (−) group. The meiotic spindle was imaged using polarized light microscopy. The rates of fertilization, high-quality day-3 embryo, day-5 blastocyst, and high-quality day-5 blastocyst were evaluated in both groups.
Results: The fertilization rates of Spindle (+) and Spindle (−) were 92.0% (450/489) and 70.0% (28/40), respectively; high-quality day-3 embryo rates were 62.9% (283/450) and 35.7% (10/28); day-5 blastocyst rates were 53.7% (205/382) and 32.1% (9/28); high-quality day-5 blastocyst rates were 29.8% (114/382) and 3.6% (1/28). Fertilization, high-quality day-3 embryo, day-5 blastocyst, and high-quality day-5 blastocyst rates were significantly higher in the Spindle (+) group than in the Spindle (−) group.
Conclusion: Spindle imaging (i.e., oocytes with a visible meiotic spindle or with not observed meiotic spindle) influences the outcome of Piezo-ICSI in human oocytes, including fertilization and embryo development. To our knowledge, this study is the first to evaluate the effect of meiotic spindle imaging on fertilization or embryo development among oocytes after Piezo-ICSI. The meiotic spindle imaging could be the indicator for the quality management of medical doctors and embryologists.
INTRODUCTION
Recent studies involving polarized light microscopy have reported a correlation between the meiotic spindle imaging of human oocytes and their fertilization rate after intracytoplasmic sperm injection (ICSI). However, these studies have focused on Conventional-ICSI, wherein beveled and spiked micropipettes are usually used, and the cytoplasm is usually aspirated into the micropipette to rupture the membrane before sperm injection (Cohen et al., 2004; Kilani et al., 2010; Montag et al., 2006; Petersen et al., 2009; Tomari et al., 2011; Wang et al., 2001). The volume of cytoplasm aspirated into the micropipette varied with every oocyte and may have an effect on the fertilization (Hiraoka et al., 2012). Therefore, this variation may also affect the correlation between the meiotic spindle imaging of human oocytes and their fertilization. Recent studies involving Piezo-ICSI (Kimura and Yanagimachi, 1995) have reported significantly higher fertilization rates as compared to the Conventional-ICSI (Fujii et al., 2020; Furuhashi et al., 2019; Hiraoka et al., 2021; Hiraoka and Kitamura, 2015; Takeuchi et al., 2001; Yanagida et al., 1999; Zander-Fox et al., 2021). One of these reasons is that there is no need to aspirate the cytoplasm into the micropipette when breaking the membrane in the Piezo-ICSI (Hiraoka and Kitamura, 2015). The Piezo-ICSI could assess more accurately the correlation between the meiotic spindle imaging of human oocytes and their fertilization after sperm injection. To our knowledge, no study has elucidated the effect of Piezo-ICSI on the association between meiotic spindle imaging in human oocytes and fertilization or embryo development. The objective of this study was to investigate more accurately the effect of the meiotic spindle imaging of human oocytes on their fertilization and embryo development by using the Piezo-ICSI.
METHODS
Patient selection
We retrospectively assessed 529 oocytes with the first polar body retrieved from 124 infertile couples (147 cycles) who attended our clinic between May 2016 and December 2018. This study included only ejaculated fresh or frozen spermatozoa and excluded surgically retrieved spermatozoa, in vitro matured oocytes, and thawed or warmed oocytes. The average age of women was 37.8±4.837.8±4.8 years and that of husband was 39.7±6.039.7±6.0 years.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its subsequent amendments or comparable ethical standards. This study was approved by the institutional review board of our institution (approved number: 17-018).
Informed consent
Informed consent was obtained from all individuals participating in the study.
Semen analysis
Before oocyte retrieval, semen analysis was performed for all couples, and low sperm count (<15<15 million sperms/mL) (Cooper et al., 2010) and low total (progressive++nonprogressive) motility (<40%<40%) (Cooper et al., 2010) were defined as male factor infertility and Piezo-ICSI program indications. Data regarding the body mass index (BMI) and smoking status of the husband were not obtained.
Ovarian stimulation
Two previously described ovarian stimulation protocols (Diedrich et al., 1994; Ferraretti et al., 2015), i.e., the clomiphene citrate protocol and the gonadotropin-releasing hormone (GnRH) antagonist protocol, were used. Vaginal ultrasound-guided follicle puncture was performed 35h after 5,000 or 10,000 IU of urinary human chorionic gonadotropin (hCG) (Mochida Pharmaceutical Company, Ltd., Tokyo, Japan) administration. Retrieved oocytes were cultured in ORIGIOⓇSequential Fert™(ORIGIO, Måløv, Denmark). Oocyte denudation was performed 37h after hCG administration, and only those with a polar body were subjected to spindle imaging and Piezo-ICSI.
Micropipette and sperm immobilization
Commercially available ultra-thin-walled Piezo-ICSI micropipettes with a flat tip were used (PINU06-20FT; PRIME TECH, Ltd., Ibaraki, Japan). A motile sperm was selected under 1,200× magnification using a microscope (Olympus IX-73 inverted microscope equipped with IX-3ICSI/IMSI, Olympus, Life Science Solutions, Tokyo, Japan) and immobilized with 2–3 piezo pulses to the sperm’s tail, while attaching the sperm’s tail to the edge of the micropipette (Hiraoka et al., 2017). After immobilization, the sperm was aspirated tail-first into the micropipette with in a 10-μL drop of 7% polyvinylpyrrolidone (PVP 7% solution; Cooper Surgical, Inc., CT, USA) on a glass-bottom dish (87-453, NIPRO, Osaka, Japan) (Hiraoka et al., 2021).
Meiotic spindle imaging
Meiotic spindle imaging was performed using polarized light microscopy (Olympus IX-73 inverted microscope equipped with IX-3ICSI/IMSI, Olympus, Life Science Solutions, Tokyo, Japan). After 38-41 h of hCG administration, oocytes were transferred into a droplet of G-MOPS PLUS™(Vitrolife, Göteborg, Sweden) medium on a glass-bottom dish (87-453, NIPRO, Osaka, Japan), and then first positioned with the polar body located at 1.5 o’clock position because this position facilitates better spindle observation under the bright-field (Fig. 1A and 2A). The meiotic spindle appears bright white (Fig. 1B: white arrow) or does not appear (Fig. 2B) in the oocyte under the polarized light microscopy. After meiotic spindle imaging, oocytes with a visible meiotic spindle comprised the Spindle (+) group and oocytes not observed meiotic spindle comprised the Spindle (−) group and were subjected for Piezo-ICSI.
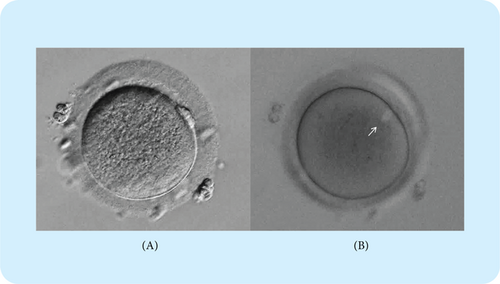
Fig. 1. Oocyte with a visible meiotic spindle.
(A) Oocyte positioned with polar body located at 1.5 o’clock position for better facilitating spindle observation under the bright-field. (B) Meiotic spindle appears bright white (white arrow) in the oocyte under the polarized light microscopy.
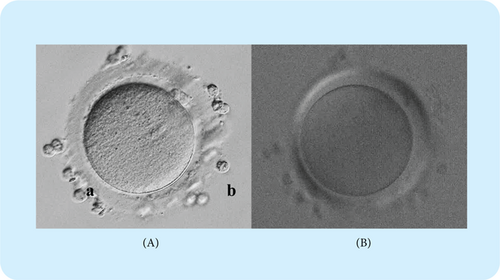
Fig. 2. Oocyte with not observed meiotic spindle.
(A) Oocyte positioned with polar body located at 1.5 o’clock position for better facilitating spindle observation under the bright-field. (B) Meiotic spindle does not appear in the oocyte under the polarized light microscopy.
Piezo-ICSI
Piezo-ICSI was performed as previously described (Hiraoka et al., 2018, 2021; Hiraoka and Kitamura, 2015). Briefly, after sperm immobilization, without oocyte deformation, the micropipette was gently placed against the zona pellucida, and piezo pulses were applied (Speed 1, Intensity 2), allowing the micropipette to rupture the zona pellucida and not the membrane. The sperm was allowed to advance until the sperm tail was near the micropipette tip and the micropipette was further advanced to stretch the membrane. The membrane was ruptured by applying a single piezo pulse (Speed 1, Intensity 2) without aspirating the cytoplasm into the micropipette, and the sperm was injected into the oocyte. To minimize the potential influence of the technique and other technical factors, the same embryologist performed all ICSI procedures.
Embryo culture and assessment
Embryo culture and assessment were performed as follows. After Piezo-ICSI, oocytes were cultured individually from day-0 (the day of oocyte retrieval was considered as day-0) until day-5 in 50-μL droplets of SAGE 1-Step™medium (ORIGIO, Måløv, Denmark) covered with mineral oil (OVOIL; Vitrolife, Göteborg, Sweden). Fertilization was assessed on day-1 (24–25h after oocyte retrieval) based on the number of pronuclei. Fertilization was defined as those with 2 pronuclei inside the oocyte. 0PN were defined as those with not observed pronucleus. 1PN were defined as those with one pronucleus inside the oocyte. 3PN were defined as those with three pronuclei inside the oocyte. On day-3, embryos originating from normally fertilized zygotes were observed. High-quality day-3 embryos were defined as those with regular blastomeres, <20% fragments, and containing at least seven cells. On day-3, 1–2 high-quality day-3 embryos were transferred or cryopreserved, and the rest were further cultured until day-5. On day-5, embryos originating from the remaining normally fertilized zygotes were observed. Day-5 blastocysts were defined as embryos classified as grade 1 or higher by Gardner’s criteria on day-5 (Gardner and Schoolcraft, 1999). High-quality day-5 blastocysts were defined as grade B or higher for both the inner cell mass and the trophectoderm (i.e., BB) by Gardner’s criteria (Gardner and Schoolcraft, 1999).
Parameters evaluated following meiotic spindle imaging
The rates of survival, fertilization, 0PN, 1PN, 3PN, high-quality day-3 embryo, day-5 blastocyst, and high-quality day-5 blastocyst were retrospectively compared between the Spindle (+) and Spindle (−) groups.
Statistical analysis
The Mann–Whitney test, unpaired Student’s t test, and Fisher’s exact test were used to determine statistical differences between groups. All probability values were two-sided. A p-value of <0.05 was considered significant. GraphPad Prism version 7 for windows (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis.
RESULTS
The table summarizes the ratio of Spindle (+) and Spindle (−), the rates of survival, fertilization, 0PN, 1PN, 3PN, high-quality day-3 embryo, day-5 blastocyst, and high-quality day-5 blastocyst after Piezo-ICSI in Spindle (+) and Spindle (−) groups. Of 529 oocytes, the number of Spindle (+) oocytes was 489 (92.4%) and the number of Spindle (−) oocytes was 40 (7.6%). Fertilization, high-quality day-3 embryo, day-5 blastocyst, and high-quality day-5 blastocyst rates were significantly higher in the Spindle (+) group than in the Spindle (−) group.
DISCUSSION
The present results show that spindle imaging (i.e., oocytes with visible meiotic spindle or not observed meiotic spindle) influences the outcome of Piezo-ICSI in human oocytes, including fertilization and embryo development.
Herein, the fertilization rate was significantly lower in the Spindle (−) than in the Spindle (+) group (70.0% vs. 92.0%), concurrent with Petersen et al., who investigated the effect of spindle imaging on fertilization rates after Conventional-ICSI in a meta-analysis and observed a lower fertilization rate in the Spindle (−) group than in the Spindle (+) group (61.5% vs. 75.6%) (Petersen et al., 2009). The 0PN and 3PN rates of Spindle (−) oocytes were significantly higher as compared to those of Spindle (+) oocytes. The invisible meiotic spindle might be in the oocyte during meiosis (immature) or might be in the oocytes starting to disassemble (overmature). Montag et al. observed the contemporary invisible meiotic spindle during meiosis (immature) (Montag et al., 2006). On the other hand, Kilani et al. reported that the maximum number of oocytes with a spindle was at an interval of 39–40.5h post-hCG when 96% had a birefringent spindle (2011). The authors also reported that the percentage of detectable spindles declined significantly after 40.5–41h post-hCG (77%) when the spindle starts to disassemble (overmature). In the case of the invisible spindle oocyte, the oocyte is immature or overmature. If the interval is before 39 h post-hCG administration, there is a high possibility that the oocyte is immature. We might have to wait for the sperm injection for about 1h. On the other hand, if the interval is after 40.5h post-hCG administration, there is a high possibility that the oocyte is overmature. We might have to inject the sperm as soon as possible. Our results demonstrated that either immature or overmature, injecting the sperm into the oocyte with an invisible meiotic spindle increases 0PN and 3PN rates that resulted in decreasing the fertilization rates. It is crucial to inject the sperm into the oocyte with a visible meiotic spindle for increasing the fertilization rate.
In the present study, the high-quality day-3 embryo rate was significantly lower in the Spindle (−) group than in the Spindle (+) group (35.7% vs. 62.9%), concurrent with Petersen et al., who investigated the effect of spindle imaging on fertilization rates after Conventional-ICSI in a meta-analysis and reported a significantly lower percentage of high-quality day-3 embryo in the Spindle (−) group than in the Spindle (+) group (28.0% vs. 37.3%) (Petersen et al., 2009). Furthermore, in the present study, the day-5 blastocyst rate was significantly lower in the Spindle (−) group than in the Spindle (+) group (32.1% vs. 53.7%), concurrent with Petersen et al., who studied the effect of spindle imaging on the day-5 blastocyst rate after Conventional-ICSI in a meta-analysis and reported a significantly lower blastocyst rate in the Spindle (−) group compared to the Spindle (+) group (28.6% vs. 50.7%) (Petersen et al., 2009). These results suggest that spindle imaging after Piezo-ICSI markedly predicts not the only oocyte fertilization but also embryonic development. It is essential to inject the sperm into the oocyte with a visible meiotic spindle for better embryo development.
In the present study, one high-quality blastocyst was obtained from the oocytes with Spindle (−) (Table 1). One possible explanation is that the spindle was overlooked during spindle imaging. Overlooking the spindle might occur when the oocyte spindle was not located near the polar body. Asa et al. reported that the meiotic spindle was not always located near the polar body (2017). In addition, Petersen et al. reported no difference in the pregnancy and implantation between the Spindle (+) and (−) groups (2009). Therefore, ICSI should be performed even for oocytes which are Spindle (−) and transfer should be considered if they develop to the blastocyst stage.
| Meiotic spindle | |||
|---|---|---|---|
| (+) | (−) | p | |
| Average age of women | 37.5±4.8 | 38.6±4.8 | N.S. |
| Average age of husband | 39.5±5.9 | 40.0±6.0 | N.S. |
| No. of total oocytes | 529 | ||
| No. of oocytes | 489 (92.4) | 40 (7.6) | — |
| No. of oocytes survived after ICSI (%) | 482 (98.6) | 38 (95.0) | N.S. |
| No. of oocytes degenerated after ICSI (%) | 7 (1.4) | 2 (5.0) | N.S. |
| No. of oocytes fertilized (%) | 450 (92.0) | 28 (70.0) | 0.0001 |
| No. of 0PN oocytes after ICSI (%) | 23 (4.7) | 6 (15.0) | 0.0164 |
| No. of 1PN oocytes after ICSI (%) | 4 (0.8) | 1 (2.5) | N.S. |
| No. of 3PN oocytes after ICSI (%) | 5 (1.0) | 3 (7.5) | 0.0172 |
| No. of high-quality day-3 embryo (%) | 283 (62.9) | 10 (35.7) | 0.0081 |
| No. of embryos cultured to the blastocyst stage | 382 | 28 | — |
| No. of day-5 blastocyst (%) | 205 (53.7) | 9 (32.1) | 0.0315 |
| No. of high-quality day-5 blastocyst (%) | 114 (29.8) | 1 (3.6) | 0.0017 |
Compared to the previous studies using the Conventional-ICSI, the present study using the Piezo-ICSI showed higher fertilization (75.6% vs. 92.0%) and high-quality day-3 embryo (37.3% vs. 62.9%) rates with Spindle (+) oocytes (Petersen et al., 2009). At the time of membrane breakage, the average volume of aspirated cytoplasm into the micropipette of Piezo-ICSI was significantly less as compared to that of Conventional-ICSI (0±0μm3 vs. 2746±940μm3) (Hiraoka and Kitamura, 2015). Cytoplasmic aspiration into the micropipette procedure might cause oocyte damage, chance of which may be lowered by using Piezo-ICSI. Therefore, the fertilization and high-quality day-3 embryo rates of the present study using Piezo-ICSI were better as compared to those of previous studies using the Conventional-ICSI. However, the day-5 blastocyst rate of the present study (53.7%) was comparable to the previous studies (50.7%) with Spindle (+) oocytes (Petersen et al., 2009). In the present study, 68 high-quality day-3 embryos were transferred or cryopreserved on day-3 out of 450 embryos, and excluded from the number of cultured to the blastocyst stage with Spindle (+) oocytes. Consequently, if all fertilized embryos were cultured, a higher day-5 blastocyst rate could be observed. This is the limitation of our study.
Tomari et al. showed that meiotic spindle size affected fertilization and embryo development after ICSI (Tomari et al., 2018). The present study only separated the oocytes into groups with Spindle (+) and Spindle (−). The spindle imaging system used in the present study is designed for mainly clinical use and could not calculate the meiotic spindle size. On the other hand, the spindle imaging system used by Tomari et al. was combined with a computerized image analysis system that enabled the calculation of the meiotic spindle size (Tomari et al., 2018). Further study would be needed to assess the relationship between meiotic spindle size and embryo development combined with Piezo-ICSI.
The Vienna consensus proposed competency and benchmark values for the ICSI fertilization rates (no. oocytes with 2PN and 2PB/no. MII oocytes injected ×100) of ≥65% and ≥80%, respectively (ESHRE, 2017). Concerning these values, it could be relevant to exclude cases including in vitro matured metaphase I oocytes, artificially activated oocytes, the use of testicular sperm, and cases of globozoospermia and asthenozoospermia (Rubino et al., 2016). The fertilization rate of oocytes with Spindle (−) was significantly 22% lower as compared to that of oocytes with Spindle (+) in the present study. It could be relevant to exclude cases where a 22% reduced fertilization rate is anticipated, including oocytes without a visualized meiotic spindle. The Vienna consensus also proposed competency and benchmark values for blastocyst development rates (no. blastocysts day 5/no. normally fertilized oocytes ×100) of ≥40% and ≥60%, respectively (ESHRE, 2017). The day-5 blastocyst rate of oocytes with Spindle (+) was significantly 21.6% higher as compared to that of oocytes with Spindle (−) in the present study. Similarly, it could be relevant to exclude cases where a 21.6% reduced blastocyst rate is anticipated, including normally fertilized oocytes without a visualized meiotic spindle. Our results suggested that the meiotic spindle observation should be discussed as one of the indicators of the key performance indicators in the future.
To our knowledge, this study is the first to evaluate the effect of meiotic spindle imaging on fertilization or embryo development among oocytes after Piezo-ICSI. The present results show that the meiotic spindle imaging has great effect on the fertilization and embryo development following the ICSI. The presence of the meiotic spindle in metaphase II (with one polar body) during ICSI could be the indicator for the quality management of medical doctors and embryologists.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDINGS
There is no funding for this study.