Combating acoustic heterogeneity in photoacoustic computed tomography: A review
Abstract
Based on the energy conversion of light into sound, photoacoustic computed tomography (PACT) is an emerging biomedical imaging modality and has unique applications in a range of biomedical fields. In PACT, image formation relies on a process called acoustic inversion from received photoacoustic signals. While most PACT systems perform this inversion with a basic assumption that biological tissues are acoustically homogeneous, the community gradually realizes that the intrinsic acoustic heterogeneity of tissues could pose distortions and artifacts to finally formed images. This paper surveys the most recent research progress on acoustic heterogeneity correction in PACT. Four major strategies are reviewed in detail, including half-time or partial-time reconstruction, autofocus reconstruction by optimizing sound speed maps, joint reconstruction of optical absorption and sound speed maps, and ultrasound computed tomography (USCT) enhanced reconstruction. The correction of acoustic heterogeneity helps improve the imaging performance of PACT.
1. Introduction
Photoacoustic computed tomography (PACT) is capable of visualizing the optical absorption of biological tissues by combining the excellent contrast in pure optical imaging and high spatial resolution in deep tissues in ultrasound imaging.1,2,3,4 The imaging modality holds great promise for a range of biomedical applications, such as early detection of cancer,5,6 inflammation evaluation,7 biopsy guidance,8,9 retinal imaging,10 and cellular imaging.11,12 In PACT, the energy of a laser pulse is absorbed by biological tissues, which will induce a rapid thermal expansion and then excite ultrasound waves. The ultrasound signals travel through the tissue and are detected by ultrasound transducers arranged in a certain detection geometry for final image reconstruction.
Conventional image reconstruction algorithms in PACT are typically based on the assumption that biological tissues are acoustically homogeneous and have a constant speed of sound (SOS) distribution.13,14,15,16,17 Since the time of flight (TOF) of ultrasound signals directly relates to the position of the target to be reconstructed, it’s essential to know the accurate distribution of SOS within the tissues. Currently, the SOS used for PACT image reconstruction is usually selected manually, which is actually different from true values. As a result, distortions and artifacts are common in PA images.
In fact, the tissues in living bodies are typically heterogeneous, violating the aforementioned constant SOS assumption. For example, in transcranial PA imaging, the acoustic reflection and refraction by the skull will cause significant PA signal distortions.18 Xu et al. found that in thermoacoustic tomography of the breast, phase distortions, i.e., TOF errors, are the major source leading to image blurring.19 Later, Deán-Ben and coworkers investigated the influence of TOF and the corresponding time shift of wave signals.20 These efforts theoretically studied the errors induced by spatially varying SOS in PA image reconstruction and show that the SOS pattern used should be sufficiently accurate to produce good imaging results.21 An example of how acoustic heterogeneity affects the final image quality is shown in Fig. 1.22 When a constant SOS of 1506m/s is assumed for image reconstruction, significant double line artifacts [Figs. 1(a) and 1(c)] occurs, which could be significantly mitigated by incorporating measured heterogeneous SOS maps [Figs. 1(b) and 1(d)].
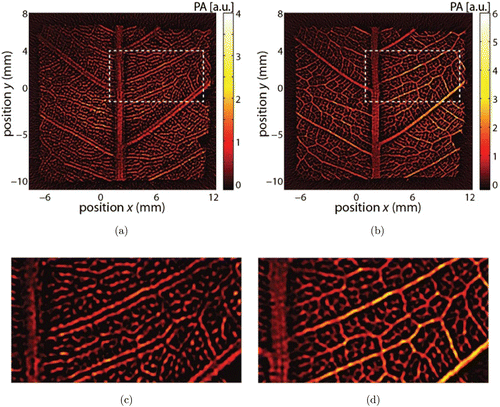
Fig. 1. An example showing how the acoustic heterogeneity may affect the final image quality. (a) PA image of a leaf skeleton reconstructed with a constant SOS of 1506m/s. (b) PA image of the same leaf skeleton reconstructed with an experimentally measured SOS map. (c) and (d) Corresponding zoomed-in views of the veins in the dashed boxes in (a) and (b). Reproduced with permission from Ref. 22.
So far, four types of approaches have been proposed to mitigate the artifacts caused by acoustic heterogeneity. They are half-time or partial-time reconstruction,23,24 autofocus reconstruction,25,26,27 joint reconstruction of optical absorption and sound speed maps,28,29,30 and ultrasound computed tomography (USCT) enhanced reconstruction,31,32,33,34 as shown in Fig. 2. The half-time or partial-time approaches reconstruct images by eliminating parts of measurement data in the time domain that are distorted by heterogeneous tissues.23,24 These approaches could achieve better image qualities based on a constant SOS map as compared with conventional reconstruction methods. The autofocus approaches also employ a constant SOS for image reconstruction as half-time methods, but the SOS value is iteratively optimized by evaluating a cost function instead of being guessed based on experience.25,26,27 The optimized SOS still deviate from real SOS distributions but may well approximate the overall characteristics of the biological tissue. Different from the previous two methods, the joint reconstruction approaches try to simultaneously extract optical absorption information and SOS distribution from PACT measurement data.28,29,30 Since the cost function constructed for optimization is generally not convex, joint reconstruction suffers from the problem of numerical instability. The USCT enhanced reconstruction approaches reconstruct PA images using SOS distribution experimentally measured by USCT and could yield accurate results.31,32,33,34 Compared with the joint reconstruction method, the USCT enhanced reconstruction approaches could yield accurate SOS maps and stable PA reconstruction. However, these approaches need extra hardware to measure the SOS map and thus increases system complexity.
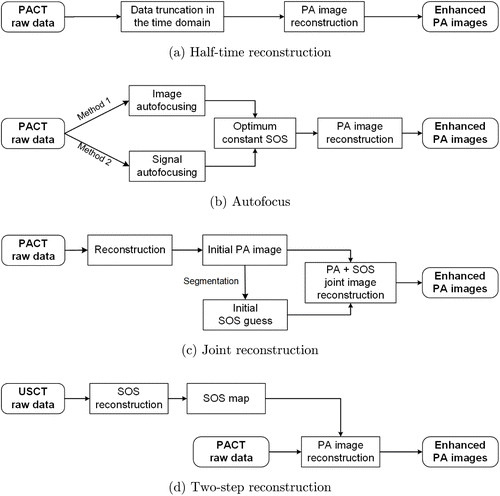
Fig. 2. Working procedure of the four methods for mitigating acoustic heterogeneity in PACT.
In this review, we surveyed the mathematical model describing the acoustic heterogeneity in PACT and state-of-the-art methods to correct acoustic aberrations. The principles and results of the established four approaches are reviewed in detail.
2. Mathematical Model
Considering PACT measurements are performed under the assumption of acoustic homogeneity, biological tissues absorb pulsed laser energy and generate acoustic waves, which are measured by ultrasound transducers located on a detection geometry Ω0. The measured acoustic pressure at the position r0 can be expressed as
Equations (1) and (3) hold only for acoustically homogeneous tissues. For tissues having different acoustic properties, images reconstructed using Eq. (3) will exhibit artifacts and distortions. Taking acoustic heterogeneities into account, Eq. (1) can be generalized as
The eikonal equation is typically adopted to track bent rays in heterogeneous media and has the form as
3. Sound Speed Correction Strategies
3.1. Half-time or partial-time reconstruction approaches
Half-time image reconstruction approaches were first proposed by Anastasio and coworkers in 2005,23 seeking to exploit redundant information in PACT measurement data. The approaches discard parts of the raw data in the time domain that are disproportionately affected by acoustic heterogeneity and only use unaffected data for image reconstruction. Therefore, it can effectively mitigate image artifacts and distortions.
The raw data g( r0, t) detected at a location r0 within the measurement geometry Ω0 can be separated into two half-time datasets g1( r0, t) and g2( r0, t) as
Figure 3 is a two-dimensional example illustrating why the half-time reconstruction approaches could mitigate image artifacts and distortions in cases of acoustic heterogeneities. The SOS map contains two concentric regions with different radii (inner disk: r1, outer ring: r0) and sound speeds (inner disk: v1, outer ring: v0, and v1≠v0). The measurement geometry is a circle with a radius of R0. The solid and dashed arc contours in red show constant acoustic path lengths to the detector for homogenous SOS map (v1=v0) and heterogeneous SOS map (v1>v0), respectively. Obviously, the difference between the solid and dashed arc contours is caused by the mismatch of SOS in the two regions and it will become more severe as the propagation time t increases. Specifically, when v0t1<R0–r1, the heterogeneity will not impact the detected signals and the data function g1(r0, t) is much less affected by acoustic heterogeneities than g2(r0, t) and g (r0, t).
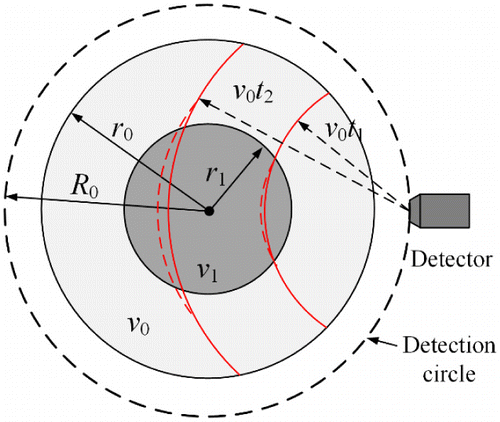
Fig. 3. Schematic showing heterogeneous sound speed distribution. Modified version of Fig. 3 in Ref. 23.
Figure 4 shows a group of simulations comparing images reconstructed by full-time and half-time approaches. The predefined SOS ratios v1/v0 in the two regions for Figs. 4(a)–4(c) are 0.9, 1.07, and 1.12, respectively. The subfigures on the left column were reconstructed using half-time raw data while those on the right were reconstructed using full-time raw data. Half-time reconstruction results have fewer artifacts and distortions compared with those reconstructed by full-time approaches.
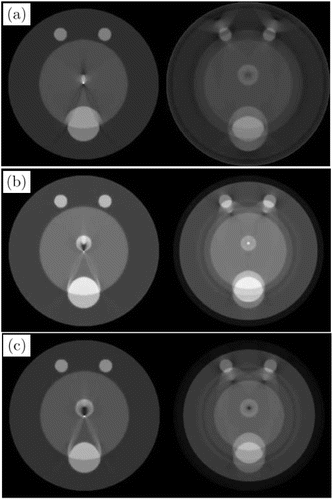
Fig. 4. Images reconstructed from simulated thermoacoustic tomography measurement data containing the effects of acoustic heterogeneities. The images in subfigures (a), (b), and (c) were reconstructed from data corresponding to acoustic speed map with v1/v0=0.9, 1.07, and 1.12, respectively. The images in the left and right panels of each subfigure were reconstructed from half- and full-time datasets, respectively. Reproduced with permission from Ref. 23.
The half-time reconstruction approaches show improved performance compared to conventional full-time reconstruction methods and were generalized to a partial-time reconstruction approach by Poudel and coworkers.24 In contrast to half-time approaches using temporally halved raw data, the partial-time method truncates raw data according to the locations of the isolated heterogeneous region and ultrasound detectors. It has better performance when the approximate sizes and locations of isolated heterogeneous structures, such as bones or gas pockets, are known.
3.2. Autofocus approaches
In PA image reconstruction, the selection of the SOS of the tissue is critical to the final image quality. Typically, the SOS used for image reconstruction is empirically chosen as a constant value (e.g., 1540m/s), which may cause blurring to finally reconstructed images.26,27 To solve this problem, autofocus methods have been proposed to optimally select the SOS for image reconstruction. These methods aim at automatically choosing the optimum SOS that can yield the best reconstruction results. The autofocus approaches usually first determine the optimum SOS by optimizing a focusing function and then reconstruct PA images based on the optimum SOS. Based on how the focusing function is constructed, autofocus approaches can be divided into image autofocus methods and signal autofocus methods.
3.2.1. Image autofocus
In practice, the SOS used for image reconstruction is manually tuned to maximize the sharpness of prominent image features, especially when the images contain vascular features. A sharper image usually contains more high-frequency components than its blurry counterparts.
Such a procedure can be automatically performed with an autofocus method,25 which selects the optimal SOS to maximize the sharpness of reconstructed PA images.35 The methods of using SOS as a focusing parameter have been used in many other modalities, such as microscopy,36 optical coherence tomography,37 and computed tomography.38 In this method, three focusing functions, that is, the Brenner gradient, the Tenenbaum gradient, and the normalized variance, can be used to autofocus the images. The Brenner gradient computes the difference between a pixel value and its neighbors two points away and can be written as
These focusing functions can be used as sharpness metrics and the SOS is automatically updated until the maximum is reached. The performance of the three cost functions was evaluated by a phantom experiment shown in Fig. 5. The result shows that the three focusing functions reach the maximum at a common SOS, where the reconstructed images have the maximum sharpness.
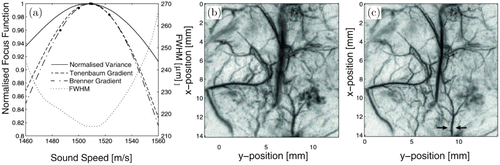
Fig. 5. Image enhancement by the autofocus method. (a) Three investigated focus metrics give the same value for the optimum SOS. (b) Defocused image reconstructed using an SOS overestimated by 5%. (c) Focused image reconstructed using the optimized SOS. Reproduced with permission from Ref. 25.
The autofocus functions are not necessarily limited to the functions listed here and can be of other types. Mandal et al. tested several different autofocus functions and classified them into three major groups, that is, intensity-based, gradient-based and edge-based measures.39 The intensity-based metrics measure the maximum pixel intensity or the maximum intensity range of the images, which is intuitive but is artifact-prone if noise or artifacts yield similar high-intensity features. The Brenner’s gradient and Tenenbaum’s gradient belong to the gradient-based measures and the normalized variance can be classified into the edge-based measures. Mandal et al. found that the edge-based measures have the best performance.39
This image sharpness maximization method can robustly determine the optimum SOS and highlights dominant image features. However, it cannot distinguish artifacts from real image details, which means that it may incorrectly maximize the sharpness of the artifacts.
3.2.2. Signal autofocus
In addition to image autofocus, Yoon et al. proposed a signal autofocus method which can also determine the optimal SOS.26 In this method, the optimal SOS is determined by maximizing a coherence factor to best focus PA signals (Fig. 6). The coherence factor used as a focusing function is computed from delay-compensated PA data as
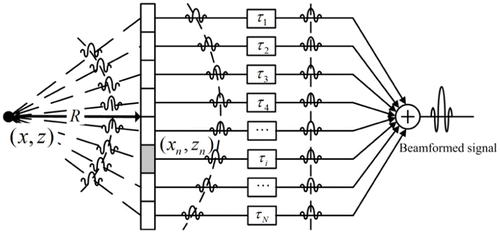
Fig. 6. Schematic showing that PA signals generated from a point-like absorber are received by a detector array when the assumed sound speed is equal to the true one. Reproduced with permission from Ref. 26.
Another focusing function that can be used to evaluate the optimum SOS is proposed by Cong et al. and can be written as27
Since image autofocus and signal autofocus both need focusing functions associated with the SOS, they fundamentally work in a similar way and can both improve the image quality by optimizing the SOS. However, since the SOS distributions within biological tissues are usually heterogeneous, the enhancement achieved by the autofocus approaches is limited especially in the case of strong SOS variations. More sophisticated approaches are required to retrieve the SOS distribution for better compensation for the acoustic heterogeneity.
3.3. Joint reconstruction approaches
Knowledge of SOS distribution of the tissues can help mitigate imaging artifacts but is difficult to obtain. Multiple methods have been developed to simultaneously reconstruct the SOS and optical absorption distributions with only PACT measurements.40,41 One algorithm that solves a Helmholtz-like PA wave equation using a finite element method (FEM) was proposed by Jiang et al. and Yuan et al. and validated with numerical simulations and phantom experiments.42,43 By introducing the FEM method to solve an accurate imaging model, this approach has better accuracy but suffers from the problem of slow computation. This FEM- based algorithm could also be extended to three-dimensional cases.44 In addition, several other algorithms were also proposed such as the TR adjoint method,45 the Radon transforms and Fourier transforms based method,40,41 and the Born approximation-based method.46 While most of the methods were only validated by numerical simulations and phantom experiments, a few were demonstrated through in vivo experiments and will be described with greater details.
3.3.1. Feature coupling based joint reconstruction
The feature coupling method was proposed by Cai and coworkers for joint reconstruction of the SOS distribution and enhancement of PA images with PA measurements using a full ring transducer array PACT system.30 This method is based on the fact that, for tissues with heterogeneous SOS distribution, any subsets of a full ring transducer array produce images with displaced features. The similarities of these images can be maximized to update the SOS distribution.
In this method, the SOS map is segmented into multiple regions with different SOS values, denoted as v = [v1, v2,…, vn]. The initial segmentation of the SOS map is achieved based on the PA image reconstructed by the half-time back-projection method23 using a predefined constant SOS. Full ring PA data are split into two parts, each part is used separately to reconstruct PA images. The features of these two images are different because the SOS maps derivate from real distributions. The similarity of the two images reconstructed by two half-ring data is maximized to get the best SOS map, i.e.,
The performance of this method has been demonstrated by in vivo experiments shown in Fig. 7. The results highlighted in the red box show that the feature coupling method can mitigate image distortions and blurring and have a better performance than the popular half-time back projection algorithm.
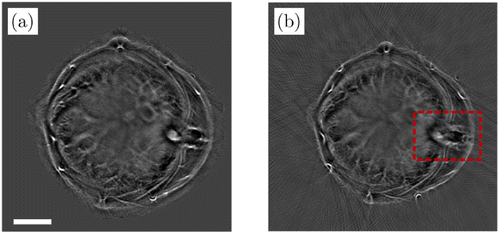
Fig. 7. PA images of a mouse liver reconstructed by the feature coupling method (a) and the half-time back projection method (b). The image in (a) has no splitting artifacts, which are present in (b). Scale bar: 5mm. Reproduced with permission from Ref. 30.
The concept of feature coupling is generic and can be applied to other detection geometries, but the detection surface division strategy should be adjusted accordingly. It should be noted that the feature coupling method optimizes the SOS distribution iteratively by maximizing the similarity measure, and therefore, a good initial guess of the SOS distribution is essential for the efficiency. In addition, if large structures with distinct features are present in the images, the performance of the feature coupling method may drop dramatically.
3.3.2. Regularization based joint reconstruction
Zhang and Anastasio reported a heuristic method to simultaneously reconstruct the SOS distribution, v( r), and the optical absorption distribution, A( r), from PACT measurements.28 The two distributions are alternatively reconstructed in an iteration process, where the optical absorption map is recovered by the full-wave iterative method47 and the SOS map is reconstructed by the use of a nonlinear optimization algorithm based on the Fréchet derivative of an objective function with respect to v( r).48,49
In this method, the distribution of SOS is unknown and is parameterized. To reconstruct A( r) and v( r) simultaneously, two-fold data redundancy is adopted.23,50 Each of the two half-time dataset contains complete information of the optical absorption map and can be used to reconstruct PA images. With complete knowledge of the SOS distribution, these two results should be the same. However, in the case of data inconsistencies, this is untrue and their differences should be minimized based on the SOS distribution. As such, both A( r) and v( r) can be recovered iteratively.
The generalized imaging model in Eq. (6) can be written in the operator form as
By alternately fixing v or A and update the other one, both distributions can be simultaneously reconstructed. Based on this model, Shan et al. proposed a deep-learning approach in 2019 using a simultaneous reconstruction network (SR-Net) to update the initial pressure and the SOS for each iteration.51In vivo experiments (Fig. 8) demonstrates the performance of a more advanced joint reconstruction method called parameterized joint reconstruction.29
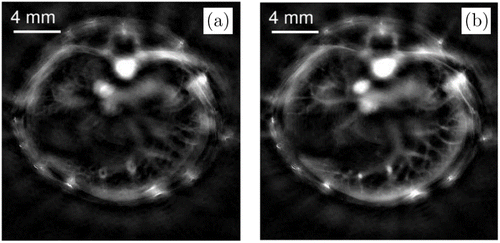
Fig. 8. Parameterized joint reconstruction. Initial pressure distributions reconstructed with a constant SOS of 1500m/s (a) and the SOS distribution obtained by parameterized joint reconstruction (b). Reproduced with permission from Ref. 29.
The numerical stability of this joint reconstruction method was studied by Huang et al.52 Since this method estimates two distributions alternatively, the errors induced in one step will affect the reconstruction accuracy in the next step, which means that errors will accumulate during the iteration process. In addition, the problem of reconstructing the SOS map, v, from estimated optical absorption map, A, is ill-conditioned, and a small fluctuation of A may cause significant variations of the reconstructed v.53Prior information or knowledge is often incorporated in the mathematical model to stabilize the numerical process; for instance, the distributions of A( r) and v( r) may have some constraints,54 and the detection surface may have specific geometrical shapes.40
This method is of significance since the results are much better than that based on a constant SOS assumption. Moreover, extra information such as USCT measurement data can be used to improve numerical stability.55
3.4. USCT-enhanced reconstruction approaches
Another strategy is to independently reconstruct the SOS map and the optical absorption distribution by USCT and PACT, respectively. Using USCT setups, high-resolution SOS maps can be recovered, which can then be used to enhance the reconstruction of optical absorption maps. There are several algorithms focusing on PA reconstruction problems in acoustic heterogeneous media, including the time reversal,56,57,58 the iterative full-wave inversion,47,59 the Neumann-series or iterative time reversal approach,60,61 the GRT,62,63 and the statistical methods.64 These reconstruction methods need measured acoustic heterogeneity as input to accurately reconstruct PA images. Some researchers try to avoid this restriction. For example, Zhang et al. proposed a method using correlation information between thermoacoustic signals to compensate for acoustic heterogeneity.65 This method compensates for travel time perturbation of thermoacoustic signals detected at locations symmetric to the origin and then reconstructs the optical absorption map. The TOF value is directly calculated from PACT signals instead of measured SOS maps.
The most difficult and critical part of USCT-enhance reconstruction approaches is how to implement the two different measurement procedures at the same time. Since USCT and PACT both detect ultrasound signals, these two measurement systems can share the same set of detectors. Efforts have been made to set up imaging systems capable of both USCT and PACT imaging. Jin and Wang integrated USCT measurements into a thermoacoustic tomography setup by adding an opposite-facing ultrasound transmitter, as shown in Fig. 9.31 In the USCT process, the transmitter and receiver work together to obtain scan data and get the SOS map of the sample. In the thermoacoustic tomography process, the sample absorbs microwave energy and generates ultrasound waves, which are detected by a receiver to reconstructed energy absorption distribution.
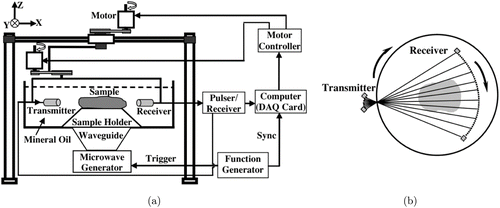
Fig. 9. Experimental set-up (a) and scanning geometry (b) of the combined USCT and thermoacoustic tomography system. Reproduced with permission from Ref. 31.
Manohar et al. proposed a similar design to achieve this purpose.32 However, instead of using an ultrasound transducer as an active transmitter, they used passive elements, i.e., carbon fiber illuminated by nanosecond laser pulses, to generate ultrasound waves for USCT measurements. This idea of leveraging laser light to generate ultrasound waves for USCT measurements was also proposed by Xia et al.33
3.4.1. Passive element enriched PACT
The passive element enriched PACT system utilized both ultrasound and PA measurements simultaneously to get accurate SOS maps and used this information to reconstruct more accurate PA images.22,66,67
The imaging model illustrated in Eq. (4) shows that the generated pressure can be seen as the projections over iso-TOF contours determined in Eq. (5), which depends on the SOS distribution v( r). In the case of homogeneous SOS distribution, the acoustic ray path is straight and the TOF is mainly decided by the SOS and travel distance. While in heterogeneous situations, the ray path is usually curved due to reflection effect as the wavefront passes through regions with different SOS. Given the SOS map estimated from USCT measurements, the high accuracy fast marching method that applies a second-order approximation is used to solve the eikonal equation.68,69,70 Then, the ray path corresponding with the shortest arrival time can be traced and used to reconstruct PA images.
Figure 10 shows the schematic of passive elements enriched PACT system and reconstructed images of ex vivo experiments. The detector is a curvilinear array containing 32 elements and used as detectors for both USCT and PACT measurements. The system performs USCT and PACT measurements in sequence and uses the SOS map obtained in USCT to correct PA images. In the USCT process, a laser pulse was used to illuminant a strand of horsetail hair to generate ultrasound pulses as source signals. The incorporating of the SOS map into PA image reconstruction process improves image quality significantly.
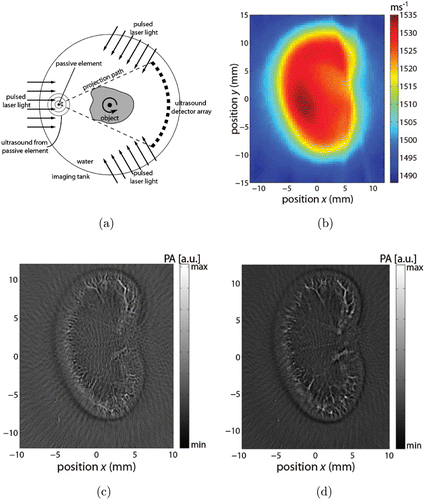
Fig. 10. Passive element enriched PACT. (a) Schematic. (b) SOS map reconstructed from the acquired passive-element measurement of the imaging area, (c) PA image reconstructed with a uniform SOS of 1540m/s, (d) SOS compensated PA image using the SOS values from (a). Reproduced with permission from Ref. 22.
3.4.2. Concurrent optical absorption and SOS imaging
Merčep and coworkers developed a transmission-reflection optoacoustic ultrasound (TROPUS) small animal imaging platform that combines both the PACT and the USCT with transmission and reflection modes.34 The system can realize multimodal imaging, including PA, SOS, acoustic reflectivity, acoustic attenuation imaging. The schematic of the TROPUS system is shown in Fig. 11. Small animals are surrounded by a ring transducer array consisting of 512 elements. The system could work in both receive-only and transmit-receive modes. In the receive-only mode, all elements are used to collect PA signals, while in the transmit-receive mode, all elements are activated to transmit ultrasound waves and receive the reflected and transmitted waves.
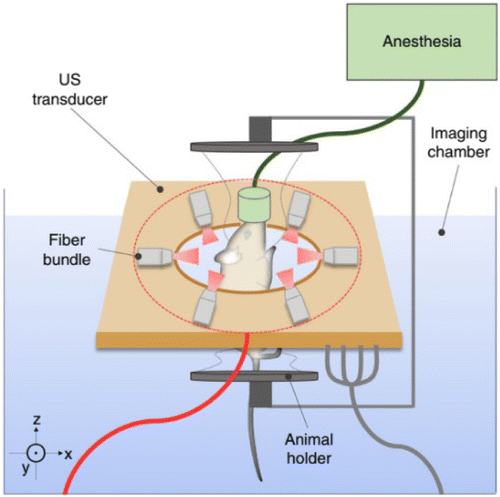
Fig. 11. Diagram of the USCT enhanced PACT imaging system. Reproduced with permission from Ref. 34.
In vivo experimental results are shown in Fig. 12. The first column is the PA images. The second column of the images is constructed from the reflection mode, while the SOS (third column) and acoustic attenuation (fourth column) are constructed from transmission mode. The results of SOS images were segmented into two parts with different SOS. These images are then incorporated into PA reconstruction to enhance the image quality.
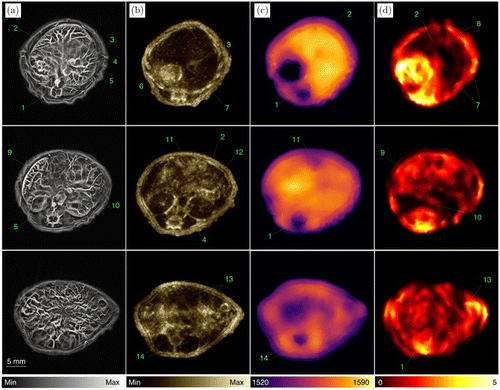
Fig. 12. USCT enhanced PACT imaging. (a) Three representative PA images of the cross-sections of a mouse. (b) Corresponding reflection-mode ultrasound images. (c) and (d) Corresponding transmission-mode ultrasound images showing the distribution of the SOS and acoustic attenuation, respectively. 1: spinal cord; 2: liver; 3: vena porta; 4: vena cava; 5: aorta; 6: stomach; 7: ribs; 8: skin/fat layer; 9: spleen; 10: right kidney; 11: cecum; 12: pancreas; 13: intestines; 14: muscle. Reproduced with permission from Ref. 34.
This imaging platform shows great potential for the combination of USCT and PACT setups. All the information that the system provides is associated because it relates to the characteristics of the same tissues. Results of one modality can be used to compensate for others, and different results can be combined to show more information.
4. Discussion and Conclusion
In summary, we reviewed four major types of approaches aiming to solve the acoustic heterogeneity problem in PACT. The half-time or partial-time approaches discard distorted measurement data in the time domain and recover PA images using conventional reconstruction algorithms with an empirically determined constant SOS. Since the data truncation step barely increases computational complexity, these approaches are simple and fast. In contrast, the autofocus approaches utilize full-time measurement data and an optimum SOS for image reconstruction. The optimum SOS is iteratively determined by evaluating a cost function describing the quality of the images or signals. Since this optimization procedure requires additional computing resources, the autofocus approaches have higher time and space complexities. Overall, the half-time/partial-time approaches and the autofocus approaches can only mitigate image artifacts and distortions to some extent in real scenarios because they are all based on the assumption that biological tissues have a constant SOS, which, however, is not true.
The joint reconstruction approaches and the USCT-enhanced approaches have improved performance in mitigating acoustic heterogeneity in PACT. The joint reconstruction approaches first segment the SOS map into multiple regions, each of which has a constant SOS, and then simultaneously recover the optical absorption map and the SOS map by optimizing a cost function using PACT measurement data alone. The joint reconstruction approaches are mathematically more complicated than the half-time and autofocus approaches and suffer from the problem of high complexity and numerical instability because the cost function may not be convex. However, this type of approach could still significantly improve image quality.29,52 The USCT-enhanced reconstruction approaches first measure heterogeneous SOS maps by USCT and then incorporate the information into the image reconstruction model to improve image quality. The SOS model in USCT-enhanced approaches is experimentally obtained rather than based on simplified assumptions as in previous three approaches and, therefore, is more accurate. USCT-enhanced reconstruction approaches are more stable but need extra setups to perform USCT measurements. Table 1 briefly summarizes the typical performance of the four approaches for acoustic heterogeneity migration in practical applications.
| Approaches | Complexity | Convex | Computational cost | Extra hardware requirement | Performance |
|---|---|---|---|---|---|
| Half-time/partial-time | Low | Yes | Low | No | Good |
| Autofocus | Moderate | Yes | Moderate | No | Good |
| Joint reconstruction | High | No.30,52 | High | No | Excellent |
| USCT-enhanced | High | Yes | High | Yes | Excellent |
It should be noted that acoustic heterogeneity induced TOF errors in PACT measurements are distinct for different detection geometries. Some correction approaches are independent of measurement geometries while others are designed for special detector apertures. For example, the half-time or partial-time approaches,23,24 joint reconstruction approaches,28,29,30 and the USCT-enhanced approaches31,32,33,34 were all developed based on ring or spherical detection geometries. Although the principles are generic and may be extended to other detection apertures, the image reconstruction process may become quite challenging. In addition, the autofocusing approaches25,26,27 enhance image quality using an optimum SOS by evaluating a focusing function and has no restrictions on the detection geometries.
The choice of the approaches for the correction of acoustic heterogeneity should depend on experimental conditions. The half-time/partial-time approaches and the autofocusing approaches are simple and fast but have limited performance. The joint reconstruction approaches have improved performance but suffer from the problem of high complexity and numerical instability. The USCT-enhanced approaches have the highest performance but require extra hardware to perform USCT measurements, which is not feasible in some practical applications. For situations where USCT measurements are difficult, the joint reconstruction approaches provide a good alternative.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (NSFC) under Grant No. 61705216, in part by the Major Science and Technology Project of Anhui Province under Grant No. 18030801138, in part by the Zhejiang Lab under Grant No. 2019MC0AB01, in part by the Research Funds of the Double First-Class Initiative, in part by the Research Fund of the USTC Smart City Institute, in part by the CAS Pioneer Hundred Talents Program, and in part by the Startup Fund of the University of Science and Technology of China (USTC).
Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.


