Preparation of chitosan-Epigallocatechin-3-O-gallate nanoparticles and their inhibitory effect on the growth of breast cancer cells
Abstract
In this paper, we prepared the nanoparticle drug carrier system between nanoparticles — chitosan and Epigallocatechin-3-O-gallate (EGCG) for breast cancer cell inhibiting application. For this drug carrier system, chitosan acts as a carrier and EGCG as a drug. Which were systematically characterized and thoroughly evaluated in terms of their inhibition rate and biocompatibility. We also did a cell scratch test and the result indicated that the chitosan-EGCG nanoparticles have inhibitory effect on the growth of breast cancer cells. The inhibition rate could reach up to 21.91%. This work revealed that the modification of nanoparticles paved a way for specific biomedical applications.
1. Introduction
Nanoparticles are highlighted as potential drug carrier systems for cancer treatment for many years. In the past, the drug carrier system was prepared by natural or synthetic polymer materials for loading drugs, and the particle size was around 1–100nm. The advantages of nanoparticles for drug carrier system is to target, the biodegradable materials that can be released slowly.1 Nano materials used in cancer therapy research are mainly used as injectable nano carriers, such as liposomes, biological targets and nano magnetic resonance imaging contrast agents.2 There are many nano medias that are used to treat cancer (including gelatin,3,4 ceramic,5 liposome6 and micelle7).
(-)-Epigallocatechin-3-O-gallate (EGCG) is a major ingredient from green tea, and possesses anticancer, anti-HIV, neuroprotective and DNA-protective properties.8 EGCG is the most effective tea polyphenol which can mop up the reactive oxygen and has a kind of compound that possesses high antioxidant properties. This compound can accomplish transformation, proliferation and inhibit tumor formation by preventing the inflammatory process.9 In the last few years, EGCG has a certain degree of growth inhibition to the cancer cells, such as on HT-29 colon cancer cells, MCF-7 breast cancer cells, BGC823 gastric cancer cells, and HepG2 liver cancer cells.10,11,12,13 EGCG showed a strong inhibition towards free radical activity during the inhibition of cell proliferation.9 Low bioavailability and in vitro experimental results have been speculated that EGCG is preferentially excluded from the gallbladder into the colon, and has no effect on the blood.14 However, the multi hydroxyl molecular structure of EGCG has become a bottleneck in its application, such as poor lipid solubility, low bioavailability, unstable under physiological environment and slow absorption in vivo.15 EGCG which belongs to polyphenols has good biological activity and is easily degraded under alkaline conditions. Dube et al. found that in the 37∘C PBS (pH 7.4), when the initial concentration was 5μμg/mL, the unencapsulated EGCG degraded by 50% after 10min and completely within 40min, degradation of 50% of encapsulated EGCG into nanospheres took 40min and complete degradation occurred within 180min.16
In recent years, biodegradable polymer nanoparticles as drug carriers have attracted more and more attention. Chitosan is one of the widely used new medicinal materials and has good biocompatibility and biodegradable, nontoxicity and sources rich in natural substances. The nanoparticles made of chitosan could improve targeting, slow release, increase drug absorption and enhance drug stability. Herein, we prepared a nanoparticle as a drug carrier system, which was formed with chitosan and EGCG.17,18 In this way, the biocompatibility and stability in vivo of EGCG could be enhanced, and also avoid excessive concentrations which cause toxicity. Then, the nanoparticles were conjugated to Folic acid (FA) to enhance targets. The prepared nanoparticles were carefully characterized and their stability, inhibition rate, and physicochemical properties were successfully evaluated.
2. Materials and Methods
2.1. Chemicals and reagents
Chitosan (CS, low molecular weight), (-)-Epigallocatechingallate (EGCG, ≥≥95%), Sodium Tripolyphosphate (STPP, 85%) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT, >98>98%) were purchased from Sigma-Aldrich. Acetic acid (CH3CO2H, 99++%) was purchased from Alfa Aesar. Folic Acid Hydrate (FA, >98.0>98.0%) and N-Hydroxysuccinimide (NHS, >98.0>98.0%) and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (EDC, >98.0>98.0%) were purchased from Tokyo Chemical Industry. Dulbecco’s Modified Eagle’s Medium (DMEM) and Phosphate Buffer Saline (PBS) were purchased from HyClone. New born calf serum (NBCS) was purchased from Sijiqing. Penicillin Streptomycin (Pen Strep) was purchased from Gibco. Deionized (DI) water used in all the studies was purified by a Milli-Q water purification system.
2.2. Synthesis and purification of nanoparticles
Chitosan was dissolved in acetic acid solution (0.175%, V/V) and was continuously stirred until the solution became clear. About 10mL of CS solution was filtered through a 0.45μμm membrane filter to which 10mg of EGCG was added and the mixture was continuously stirred with a magnetic stirrer for 30min. Then 2.5mL of STPP solution of 0.1% (w/v) dropwise was added till the solution changed from transparent to translucent, and then magnetically stirred for 4h to prepare CS-EGCG nanoparticles suspension. The above operations were conducted at room temperature.
2.3. Conjugation of nanoparticles with folic acid (FA)
Folate receptor (FR) has proved to be a tumor associated antigen when combined with drugs conjugated with FA into cells with very high affinity by the endocytic pathway. Preparation of 2mgmL−1−1 of EDC⋅⋅HCl solution and 4mgmL−1−1 of NHS solution were postponed. Add 80μμl of EDC⋅⋅HCl solution drip into the 4mL of sample solution with magnetic stirring for 5min. Continue adding 80μμl of NHS solution along with magnetic stirring for 20min. A total of 2mgmL−1−1 of FA has been prepared by dissolving DMSO addition into the above solution. FA-CS-EGCG nanoparticle suspension is obtained and kept at room temperature for further use.
2.4. Characterization of nanoparticles
Particle size distribution was measured by Zetasizer Nano (Malvern Instruments Nano ZS90, Worcestershire, UK). Transmission electron microscopy (TEM), which projects the electron beam by gathering and accelerating on very thin samples, electrons and the atoms in the sample collide to change direction, resulting in a solid angle scattering. TEM images were obtained using G20 S-TWIN transmission electron microscope (FEI Tecnai, Hillsboro, USA). Fourier Transform infrared (FT-IR) spectroscopy can be used to detect various chemical molecules in different kinds of chemicals and has a very high rate of identification at the same time. FT-IR spectra were recorded on Nicolet iS50 FT-IR spectrometer (Thermo Scientific, Massachusetts, USA). In the FT-IR characterization, the nanoparticle powder was mixed with potassium bromide (KBr) at 1:10 (w/w), ground and compressed into tablets.
2.5. Cell uptake of CS-EGCG nanoparticles and viability study
Human breast cancer cell line MCF-7 was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone). The medium was supplemented with 10% (v/v) New born calf serum (NBCS, Sijiqing) and penicillin streptomycin (100μμgmL−1−1, Pen Strep, Gibco). Cells were cultured at 37∘C in a carbon dioxide incubator with 5% CO2.
For cell imaging, Rhodamine 6G (R6G) was used to mark the nanoparticles, MCF-7 cells were treated with PBS, CS-EGCG or FA-CS-EGCG nanoparticles and incubated for 4 h. Before imaging, cells were washed with PBS and fixed with 5% formaldehyde. Fluorescence imaging was observed by fluorescent inverted microscope (Leica DMI 3000B, Solms, GER).
Cell activity test was performed by cell scratch test in this paper. All equipment should be sterilized before operation, the ruler and pen marker should be under UV irradiation for 30min in a super-clean bench. Use the marker pen in an uniform manner to draw a line every 0.5–1cm behind the cell culture plate with 6holes, where each hole passes through at least five lines. Adding about 5×1055×105 cells in the holes can make the cells fill the hole by the end of the night. On the second day, Reep the pipette tip scratches perpendicular to the line marked on the back of the cell culture plate, keeping pipette tips vertical. Wash the cells with PBS three times in order to remove the scratched out cell, and then add the serum-free medium. The cells were cultured at 37∘C in a carbon dioxide incubator with 5% CO2. They were observed carefully and photos were taken every 12h. We can calculate the inhibition rate based on the equation19 :
3. Results and Discussion
3.1. Synthesis and characterization of nanoparticles
Chitosan is a kind of polymer, which possesses obvious biological characteristics, such as biodegradability, biocompatibility, low toxicity and so on. Table 1 shows the utility of chitosan in combination with some drugs. At the same time, drug surface modification by chitosan can improve the transfection efficiency better than nanometer gene therapeutic agents.
| Carrier | Drug | Size | Effect | Ref. |
|---|---|---|---|---|
| Chitosan | Doxorubicin | 292±±42nm | Reduce the toxic and side effects | 20 |
| Chitosan | Insulin | 250–400nm | Prevent insulin to disintegrate by gastric acid | 21 |
| Chitosan | Fluorouracil | 1–5μμm | Slow-release | 22 |
| Chitosan | Retinol | 50–200nm | Improve stability | 23 |
| Chitosan | Vitamin C | 216–288nm | Controlled release | 24 |
As illustrated in Fig. 1, firstly, we applied CS as the carrier, secondly loaded the EGCG into the CS, then we obtained the CS-EGCG nanoparticles. In order to make the nanoparticle target cancer better, we combined the FA with CS-EGCG nanoparticles.
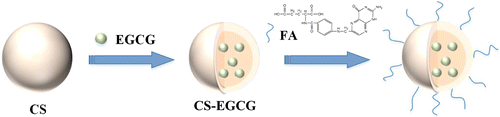
Fig. 1. Schematic of CS-EGCG nanoparticle and FA-CS-EGCG preparation.
Figure 2 shows the FTIR spectrum of chitosan, EGCG and CS-EGCG nanoparticles. EGCG has obvious vibration peaks located at 1690cm−1−1 and 1608cm−1−1 as shown in Fig. 2(a). Chitosan has two characteristic peaks located at 1612cm−1−1 and 1056cm−1−1. The formation of new peaks located at 1538, 1446, 1376, and 1207cm−1−1 was observed in the spectrum of CS-EGCG nanoparticles. A part of these vibration peaks disappeared in the synthesis of CS-EGCG nanoparticles confirming the encapsulation of EGCG in the nano-carrier. Figure 2(b) shows the FTIR spectrum of FA and FA-CS-EGCG. There was a strong FA absorption peak located at 1695cm−1−1. The new peak of FA-CS-EGCG at 1381cm−1−1 and 1071cm−1−1 contributed to the interaction between FA and chitosan, caused by the formation of N–H bending vibrations between the −−CO group of FA and the −−NH group of chitosan. These results indicate that the formation of FA–CS–EGCG polymer was successful.25,26
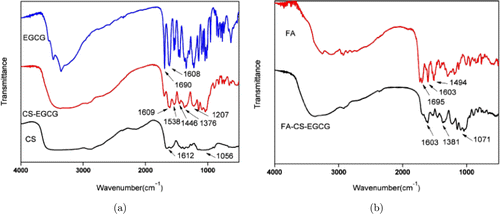
Fig. 2. FT-IR spectra of (a) EGCG, CS and CS-EGCG, (b) FA and FA-CS-EGCG.
To further prove this nanostructure, we introduced the analysis of TEM images and Selected Area Electron Diffraction (SAED). Through a joint analysis of TEM and SAED, we can achieve synchronization analysis of micro area phases and crystal structure. Through TEM photographing of the high-rate micro region of the image, combined with the SAED pattern information, we can not only observe the micro morphology information of the material but can also determine the crystal structure and crystal phase composition of the region. In order to achieve acquisition and analysis sample details in multiangle, the reliability of the sample analysis was improved.27 The representative TEM images of only CS nanoparticle can be seen in Fig. 3(a). The CS nanoparticle is nearly spherical in shape and its diameter is estimated to be 200nm. Its SAED image is present in Fig. 3(d), but we cannot see the lattice fringes from the image. EGCG and CS-EGCG TEM figures are shown in Figs. 3(b) and 3(c), and the diameter of CS-EGCG is estimated to be 60nm and their SAED images are shown in Figs. 3(e) and 3(f), the multiple lattice fringes are both observed in the SAED images of EGCG and CS-EGCG, and their lattice spacing is almost 0.24nm. From the above results, we can see that EGCG has successfully loaded into CS, the nanoparticle drug carrier.
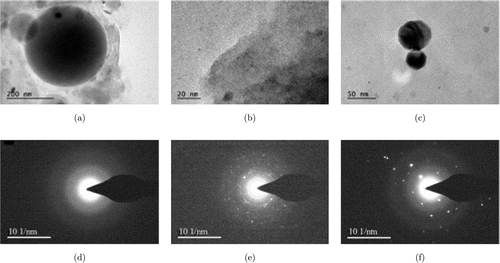
Fig. 3. TEM images of (a) CS nanoparticles, (b) EGCG (c) CS-EGCG nanoparticles and the SEAD image of (d) CS nanoparticles, (e) EGCG and (f) CS-EGCG nanoparticles.
The dynamic light scattering is an important factor for nanoparticle dispersible and colloidal stability evaluation. As shown in Fig. 4, we observe that there was no severe aggregation after the process of FA bioconjugation. The average hydrodynamic size of CS-EGCG nanoparticles was determined to be 164.2nm, which increased to 342nm after being conjugated with FA. Both DLS and TEM results showed good agreement in providing the size of nanoparticle systems.
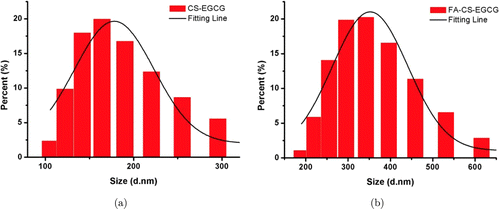
Fig. 4. Hydrodynamic size distribution of (a) CS-EGCG and (b) FA-CS-EGCG in aqueous suspension measured by dynamic light scattering (DLS).
It is important for the DLS data to be slightly different from the TEM images. This is reasonable because the hydrodynamic diameter obtained from DLS images takes into account the polymeric coating and hydration layer surrounding the particles, whereas TEM represents only the electron-rich particles.
Colloidal stability of nanoparticles is a very important factor to be considered when one is using them for the biological application such as drug carrier. As shown in Fig. 5, the colloidal stability of the prepared nanoparticles solution is evaluated at different pH values for six days at room temperature. We can see that the CS-EGCG nanoparticles are relatively stable at pH of 4, 7 and 9 and the average particle size is maintained between 190nm and 220nm. There was no precipitate, aggregation and deposition of CS-EGCG nanoparticles over a long period of time showing that they possess good stability and biocompatibility. It indicated that the colloidal stability of CS-EGCG nanoparticles was not affected by different pH values. These nanoparticles can be used as drug carriers in biological environment.
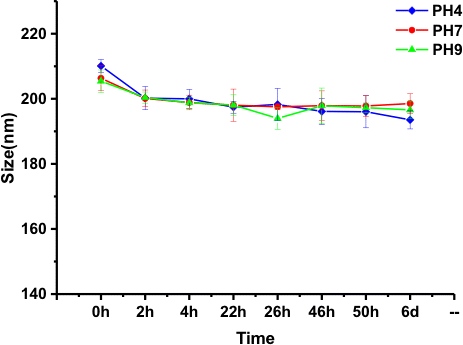
Fig. 5. Colloidal stability study of CS-EGCG nanoparticles at different pH values (4, 7, 9) for six days.
3.2. Fluorescence imaging of MCF-7 cells
The selective uptake of different nanoparticle formulations by MCF-7 cells is monitored by fluorescence microscopy (in Fig. 6). For cell imaging, in order to detect the luminescent signal from the labeled cells, we applied R6G in conjugation with nanoparticles. MCF-7 cells are separately treated with PBS, 100μμgmL−1−1 CS-EGCG or FA-CS-EGCG nanoparticles for 4h before they are examined under the microscope. As shown in Fig. 6, three groups of cell growth are in good condition. The cell morphology was normal MCF-7 polygon, and there was no damage to the cell morphology with 4h treatment. The FA-mediated targeting can be observed by the R6G luminescent signal from the labeled MCF-7 cell, and the FA-CS-EGCG nanoparticles were more uniformly distributed than the CS-EGCG nanoparticles in the vesicles within the cell. It indicates that CS-EGCG enters cells more easily after conjugating with FA.
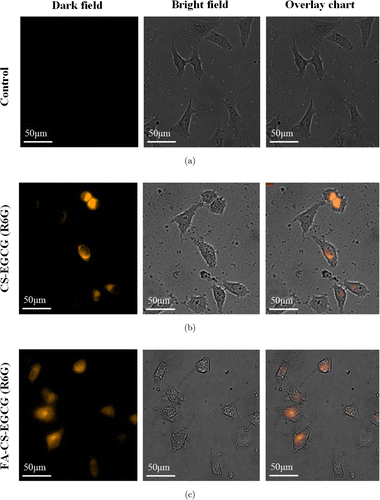
Fig. 6. Inversed fluorescent microscope images of MCF-7 cell culture treated with (a) Control, (b) CS-EGCG and (c) FA-CS-EGCG.
3.3. Cell inhibition rate studies of nanoparticles
For the cell inhibition rate study, we did a cell scratch test to evaluate the inhibition rate of breast cancer cell of the CS-EGCG nanoparticles. Cell scratch test is one of the methods to measure the motion characteristics of tumor cells. It uses reference cell injury healing model in vitro, scratches in monolayer cells of in vitro culture, and then adds the drug to observe its ability to inhibit tumor cell migration. When the drug was added, the cell migration was inhibited due to the action of the drug, and the cells maintained their original migration ability when the drug was not added.
As shown in Fig. 7, the breast cancer cell growth rate decreased with the increasing EGCG concentration. Compared to the control group, when the concentration of EGCG is 0.15mM and 0.30mM the cell growth inhibition rate is 18.07% and 21.91% after 72h incubation. It indicated that, after treatment with CS-EGCG, a significant inhibition of the cell proliferation and the migration of the cells could be observed. With increase in EGCG concentration, the morphology of breast cancer cells was severely damaged.
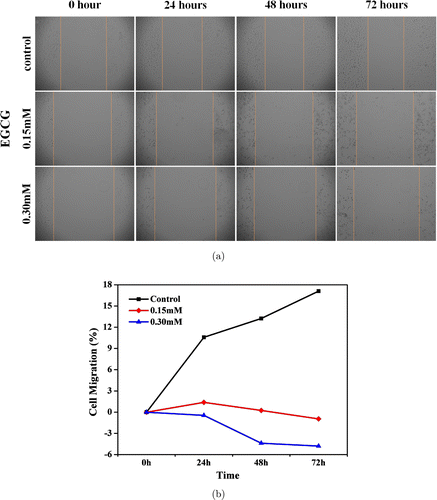
Fig. 7. Wound healing assay in MCF-7 cell culture treated with different concentration. (a) Inversed fluorescent microscope images of the wound healing process monitored for 72h post-treatment. (b) Quantitative evaluation on the percentage of the wound window closed after treatment.
4. Conclusion
In summary, we have synthesized CS-EGCG nanoparticle as a drug carrier system. The colloidal stability, FTIR spectrum and bioimaging of the nanoparticles have been systematically studied. The result showed excellent colloidal stability of CS-EGCG nanoparticles without being precipitated within six days under different pH values. This study revealed that CS-EGCG nanoparticle possesses excellent stability and biocompatibility for biological applications, and exhibits an excellent inhibitory effect on the growth of breast cancer cells.
Conflict of Interest
We declare that the authors have no competing interests as defined by Journal of Innovative Optical Health Science, or other interests that might be perceived to influence the results and discussion reported in this paper.
Acknowledgments
The authors would like to acknowledge the support of the National Natural Science Foundation of China (NSFC Nos. 61722508 and 11305020), Nanophotonics and Biophotonics Key Laboratory of Jilin Province, P. R. China (20140622009JC) and (14GH005). Y. Liu and S. Hu contributed equally to this work.