Ambulatory chest physiotherapy in mild-to-moderate acute bronchiolitis in children under two years of age — A randomized control trial
Abstract
Objective: The aim of this study was to compare the role of a chest physiotherapy (CP) intervention to no intervention on the respiratory status of children under two years of age, with mild-to-moderate bronchiolitis.
Methods: Out of 80 eligible children observed in the Emergency Room, 45 children completed the study with 28 randomized to the intervention group and 17 to the control group. The intervention protocol, applied in an ambulatory setting, consisted of combined techniques of passive prolonged slow expiration, rhinopharyngeal clearance and provoked cough. The control group was assessed with no chest physiotherapy intervention. The efficacy of chest physiotherapy was assessed using the Kristjansson Respiratory Score at the admission and discharge of the visit to the Emergency Room and during clinical visits at day 7 and day 15.
Results: There was a significant improvement in the Kristjansson Respiratory Score in the intervention group compared to the control group at day 15 [1.2 (1.5) versus 0.3 (0.5); -value, in the control and intervention groups, respectively], with a mean difference (95% CI) of ( to ).
Conclusion: Chest physiotherapy had a positive impact on the respiratory status of children with mild-to-moderate bronchiolitis.
Clinical Trial Registration:https://clinicaltrials.gov/ct2/show/NCT04260919.
Introduction
Acute bronchiolitis is the most common lower respiratory tract infection in infants and children younger than two years of age. It occurs in a seasonal pattern with significant burden on infants, their families and the healthcare system.1 Acute bronchiolitis is usually a self-limited condition, characterized by acute inflammation, oedema and necrosis of the epithelial cells lining small airways, and increased mucus production. Clinically, it is typically characterized by a 2–3 day prodrome of coryza and cough, followed by signs of respiratory distress as nasal flaring, tachypnoea and chest retractions, with rales, fine crackles or wheezing on auscultation.2,3 The severity of the acute episode of bronchiolitis is usually established by a physician, based on clinical findings.2,4 In most cases, the disease is mild to moderate and can be treated at home; however, 1–3% of the cases develop severe disease and require hospitalization.5,6 In up to 85% of the hospitalized cases, the disease is caused by respiratory syncytial virus (RSV).2,4
The best treatment approach for children hospitalized with bronchiolitis remains controversial and there is still substantial variation regarding the practices followed by physicians. Current scientific guidelines recommend that the standard treatment of choice should be supportive, which includes supplemental oxygen when needed, appropriate fluid therapy and overall a “minimal handling approach”.1,7 According to guidelines and systematic reviews published to date, chest physiotherapy (CP) is not recommended as a standard treatment for bronchiolitis.2,4,8,9
However, it is of note that most of these recommendations and reviews are based on studies which applied classical CP methods, such as clapping, percussion or vibration technics, to hospitalized patients with bronchiolitis. To date, very few studies have been conducted with the application of more modern CP techniques in hospitalized patients.10,11,12 Furthermore, although the majority of bronchiolitis cases are mild to moderate, most of the studies conducted so far focused on more severe cases.
The few studies that tested modern CP techniques in acute bronchiolitis patients, such as prolonged slow expiration (PSE) and rhinopharyngeal retrograde clearance (RRC), presented favorable results, suggesting that the use of CP techniques in the management of bronchiolitis could be considered, if not recommended, in some cases, depending on the severity of the disease.10,12,13 To the best of our knowledge, no randomized study has yet tested the efficacy of any kind of CP, neither classical nor more recent technics, in mild-to-moderate acute bronchiolitis cases managed as outpatients.
The main objective of the study was to analyze the role of a modern CP intervention to no intervention on the respiratory status of children under two years of age, with mild-to-moderate bronchiolitis.
Methods
Patients
The inclusion criteria considered children up to two years of age admitted at the Paediatric Emergency Department (PED) with a diagnosis of acute bronchiolitis and clinical conditions that allowed the child to be discharged home after acute management in the PED.
The diagnosis of acute bronchiolitis was established by the attending physicians, based on the classical clinical signs and symptoms, including the presence of coryza, cough, fever, chest hyperinflation, increased respiratory rate (RR) or other signs of respiratory distress, wheezing or wheezing with crackles on auscultation, and changes of feeding routine.2,4
Exclusion criteria were: (1) Severe bronchiolitis: RRbpm or 50bpm (in children younger than six months or older, respectively), global retractions, apnea, nasal flaring, oxygen saturation (SpO2) %, lethargy, dehydration and abnormal peripheral perfusion; (2) need for admission to the inpatient department; and (3) presence of comorbidities, namely prematurity, chronic pulmonary or neuromuscular diseases, congenital heart diseases, trisomy 21 or other congenital malformations.
Settings
The study was conducted during two epidemic seasons, from December to March of 2011 and 2012, at the PED of a Northern Portugal tertiary hospital [Centro Hospitalar Universitário de São João, Porto (CHUSJ)].
All children fulfilling the inclusion criteria and none of the exclusion criteria were invited to participate and their parents/legal guardians were given detailed information on the study protocol provided by the responsible physiotherapist of the study.
The trial was registered at ClinicalTrials.gov (NCT04260919) and was reported according to CONSORT guidelines.14
Randomization was conducted by permuted blocks.15 Allocation envelopes were stored in sequentially numbered (from 1 to 6), opaque, sealed envelopes, prepared by a person not involved in the study, and opened after the inclusion of a new case.
Observations and study intervention
All children were observed in a quiet environment, while awake and not crying, and were submitted to a standard protocol consisting of clinical demographic data collection and assessment of oxygen saturation using pulse oximetry and of the Kristjansson Respiratory Score (KRS) to quantify the severity of the respiratory status of the child.16,17,18 Although Wang Respiratory Score (WRS) is a more widely used score, studies comparing it with KRS show that this has better interobserver reliability, a very important aspect to this study.17,18 This assessment was attributed to each child, at PED admission, at PED discharge and at day 7 and day 15.
Children allocated to the intervention group (IG) underwent a standard intervention CP protocol between PED admission and discharge, and after PED discharge. The protocol was performed by a single physiotherapist and consisted of a 20-min session taking place during working days in the first week (five sessions), and every other day during the second week (three sessions), with a total of eight sessions. All sessions were carried out, as outpatients, in the Physical Medicine and Rehabilitation Department of CHUSJ. A series of exams were carried out in every session, namely the CP protocol, repeated lung auscultation and continuous monitoring of peripheral oxygen saturation levels and heart rate (Nonin Medical, Inc., Model 3100, Plymouth, MN, USA). If desaturation SpO% or signs of severe respiratory distress, such as global retractions, cyanosis or nasal flaring, fever, irritability or lethargy were identified by the CP protocol initiation or occurred during the session, the intervention was immediately canceled and medical evaluation was requested.19
The CP protocol included the application of three different techniques: PSE, RRC and provoked cough (PC). PSE was achieved by applying bimanual pressure over the thoracic cage and the abdomen at the beginning of the expiratory phase down to the residual volume and maintained for 2–3 respiratory cycles.10,20 RRC was accomplished by instillation of isotonic saline solution (0.9% NaCl) through the nostrils, followed by mouth closure, forcing inspiration through the nasals cavities and removing secretions from this area to the oropharyngeal cavity.21,22,23 These maneuvers were carried out during consecutive breathing cycles in order to promote the mobilization of secretions towards the proximal airways. This stimulated the mechanical receptors and made the children cough spontaneously.10,20 If no spontaneous coughing occurred, coughing was triggered by PC, accomplished by smoothly pressuring the trachea at the level of the suprasternal notch at the end of the inspiration.10,20,21
Children from the control group (CG) were not submitted to any CP protocol and were assessed at the same moments of evaluation (admission/discharge of PED, day 7 and day 15). Both groups received similar recommendations on general support measures and were medicated, as needed, by the physician responsible for the child discharge from the PED. The assessment with KRS and SpO2 in the PED was performed by the physician responsible for the initial assessment of the children. During the subsequent two weeks, CG was assessed by the physiotherapist responsible for the study and IG was assessed by a physician of the Physical Medicine and Rehabilitation Department of CHUSJ.
Considering the nature of this study, a double-blind assessment was not possible, as both physiotherapist and parents were aware of the intervention.
Outcome measures
The primary outcome was respiratory status, assessed by KRS on day 15. The secondary outcome was respiratory status, assessed by KRS on day 7. This is a five-item score which includes respiratory rate, chest recessions/retractions, breath sound/wheezing, skin color and general condition. Each clinical sign is scored from zero to two and the total score ranges from 0 to 10, with the severity being established as the total score increases (Table 1).16,17,18
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Respiratory rate (breaths/min) | 40–60 | ||
| Chest recessions | None | Moderate (costal diaphragmatic) | Severe (as in 1 plus rib and jugular retraction) |
| Breath sound | Vesicular | Wheeze / rhonchi/rales | Severe wheeze / rhonchi/rales |
| Skin color | Normal | Pallor | Cyanosis |
| General condition* | Not affected | Moderately affected | Severely affected |
Statistical analysis
Data was analyzed using IBM SPSS Statistics version 23.0. Continuous variables are presented as mean and standard deviation. To check the homogeneity of groups, the -test was used for independent samples on the continuous variables and Qui-square test for categorical variables. Differences between groups were evaluated using ANOVA. Statistically significant differences () were noted with an asterisk ().
The assumptions of ANOVA for repeated measures include normality, homogeneity of variances, homogeneity of the matrix of variances and sphericity.24 In this study, normality, skewness and kurtosis values were verified, in order to validate the results obtained from the statistics.25 The absolute values of skewness and kurtosis can be slightly higher than (1.96; 1.96), namely (3; 3) and (7; 7), respectively, without any problem in the analysis of linear models, as in the case of ANOVA.24,26
After verifying each assumption, it was possible to apply ANOVA for repeated measures, proceeding with Bonferroni’s post-hoc tests.25 The main factors were tested by SPSS, while multiple comparisons were obtained by Syntax.
Ethics
The study was approved by the Ethics Committee of CHUSJ, and complied with both the Helsinki Declaration and the current national legislation. Verbal and written consent were obtained from caretakers on behalf of all children enrolled in the study.
Results
During the study period, a total of 105 children were assessed for eligibility to participate in this study, but 15 fulfilled the exclusion criteria (prematurity: 5, chronic pulmonary diseases: 2, chronic neuromuscular disease: 2 and congenital heart disease: 6) and 10 were admitted into hospital because of the severity of the respiratory distress. The remaining 80 cases were randomly assigned to the IG () and to the CG (). In the end, a total of 45 children completed the study (, IG; , CG) (Fig. 1). Loss to follow-up was mainly due to non-attendance at the scheduled re-evaluation sessions, 10/42 (23.8%) in IG and 20/38 (52.6%) in CG or by indication to be withdrawn from the study due to hospital admission following clinical worsening (IG, ; CG, ) or other clinical problems [gastroenteritis or vascular disease (IG, )]. The baseline demographics of children, parents’ educational level and clinical characteristics are described in Table 2. No differences were found between the groups in the baseline demographic or clinical variables.
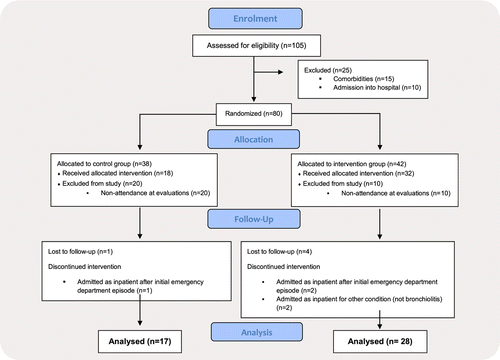
Fig. 1. Screening, random assignment and follow-up of intervention and control groups.
| Control group () | Intervention group () | -Value | |
|---|---|---|---|
| Demographics characteristics | |||
| Male gender | 14 (82.4) | 20 (7.4) | 0.408 |
| Age (months) | 11.5 (6.737) | 9.3 (5.463) | 0.228 |
| Educational level | |||
| Father | 9 (3.204) | 9.15 (4.504) | 0.909 |
| Mother | 10.35 (3.101) | 10.54 (4.238) | 0.878 |
| Clinical characteristics | |||
| First episode of bronchiolitis (yes) | 11 (64.7) | 14 (50) | 0.372 |
| Respiratory rate (cpm) | 13 (76.5) | 19 (67.9) | 0.737 |
| Peripheral oxygen saturation (%) | |||
| At admission | 95.5 (1.505) | 96 (2.085) | 0.254 |
| At discharge | 96.7 (2.144) | 97.7 (1.517) | 0.082 |
| Medication in emergency department* | |||
| Salbutamol | 16 (94.1) | 26 (92.8) | |
| Hypertonic solution | 1 (5.9) | 2 (7.2) | |
| Ipratropium bromide | 7 (41.1) | 19 (67.9) | |
| Betamethasone | 5 (29.4) | 4 (14.3) | |
| Ibuprofen | 1 (5.9) | 0 (0) | |
| Antibiotic | 0 (0) | 1 (3.6) | |
| No medication | 0 (0) | 1 (3.6) | |
Concerning the four assessments, there was a trend towards a significant improvement in KRS at day 7, where the IG shows better results compared to the CG [mean difference (95% CI) 0.6 (1.3–0.01); -value ] which became a significant improvement by day 15 [mean difference (95% CI) 0.9 (1.6 to 0.3); -value] (Table 3).
| KRS | Assessment | Intervention group mean (SD) | Control group mean (SD) | Mean difference (95% CI) | -Value |
|---|---|---|---|---|---|
| Admission | 3.4 (1.3) | 3.3 (1.3) | 0.1 (0.7–0.9) | 0.805 | |
| Discharge | 1.9 (0.9) | 1.4 (1.1) | 0.6 (0–1.2) | 0.058 | |
| Day 7 | 1 (0.8) | 1.6 (1.3) | 0.6 (1.3–0.01) | 0.054* | |
| Day 15 | 0.3 (0.5) | 1.2 (1.5) | 0.9 (1.6 to 0.3) | 0.005* |
When each assessment was compared with the following assessments, the IG had a significant improvement in the KRS score over time indicating a resolution of respiratory severity (Table 4). The CG did not show a significant improvement in KRS score when comparing discharge to the following assessments (Table 4).
| Group | Assessment () | Assessment () | Mean difference () (95% CI) | -Value |
|---|---|---|---|---|
| Control Group () | Admission | Discharge | 1.9 (1.1–2.8) | 0.001* |
| Day 7 | 1.7 (0.7–2.7) | 0.001* | ||
| Day 15 | 2.1 (1.1–3.1) | 0.001* | ||
| Discharge | Day 7 | 0.2 (1.2–0.8) | 1 | |
| Day 15 | 0.2 (0.6–1) | 1 | ||
| Day 7 | Day 15 | 0.4 (0.5–1.3) | 1 | |
| Intervention Group () | Admission | Discharge | 1.5 (0.8–2.1) | 0.001* |
| Day 7 | 2.4 (1.6–3.2) | 0.001* | ||
| Day 15 | 3.1 (2.4–3.9) | 0.001* | ||
| Discharge | Day 7 | 1 (0.2–1.7) | 0.008* | |
| Day 15 | 1.7 (1.1–2.3) | 0.001* | ||
| Day 7 | Day 15 | 0.7 (0–1.4) | 0.053 |
Table 5 indicates the individual score items for the KRS at admission and day 15. While there was no significant difference in any individual parameter between the groups at admission, there were significant improvements at day 15 in the IG compared to the CG in respiratory frequency and chest retractions.
| Admission (%) | Day 15 (%) | ||||||
|---|---|---|---|---|---|---|---|
| Control group () | Intervention group () | -Value | Control group () | Intervention group () | -Value | ||
| Respiratory frequency | 40 | 4 (23.5) | 9 (32.1) | 0.515 | 9 (52.9) | 24 (85.7) | 0.016* |
| 40–60 | 10 (58.8) | 17 (60.7) | 8 (47.1) | 4 (14.3) | |||
| 3 (17.6) | 2 (7.1) | 0 (0) | 0 (0) | ||||
| Chest retractions | None | 3 (17.6) | 1 (3.6) | 0.091 | 13 (76.5) | 28 (100) | 0.027* |
| Moderate | 14 (82.4) | 23 (82.1) | 3 (17.6) | 0 (0) | |||
| Severe | 0 (0) | 4 (14.3) | 1 (5.9) | 0 (0) | |||
| Breath sounds | Vesicular | 0 (0) | 1 (3.6) | 0.428 | 13 (76.5) | 26 (92.9) | 0.220 |
| Wheeze and rales | 15 (88.2) | 26 (92.9) | 3 (17.6) | 2 (7.1) | |||
| Severe wheeze pronounced rales | 2 (11.8) | 1 (3.6) | 1 (5.9) | 0 (0) | |||
| General condition | Not affected | 11 (64.7) | 17 (60.7) | 0.728 | 15 (88.2) | 27 (96.4) | 0.285 |
| Moderately affected | 6 (35.3) | 10 (35.7) | 2 (11.8) | 1 (3.6) | |||
| Severely affected | 0 (0) | 1 (3.6) | 0 (0) | 0 (0) | |||
| Dermal coloration | Normal | 16 (94.1) | 25 (89.3) | 0.581 | 17 (100) | 28 (100) | — |
| Pallor | 1 (5.9) | 3 (10.7) | 0 (0) | 0 (0) | |||
| Cyanosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
An important point to mention is that there were zero cases in the intervention group that experienced clinically relevant side-effects.
Discussion
To the best of our knowledge, this is the first study evaluating the effects of modern CP techniques in mild-to-moderate bronchiolitis in an outpatient setting. In this study, aiming to analyze the impact of an ambulatory modern CP intervention based on PSE associated with RRC and PC in the recovery of mild-to-moderate acute bronchiolitis, in children under the age of two years, we found that the respiratory status, assessed by a respiratory score, KRS, on day 15, significantly improved in children submitted to the tested intervention, when compared to the CG.
At the second assessment, at emergency room discharge, after only one intervention in the PED, the IG showed already a trend towards a better clinical status, when compared to the CG. At the end of the intervention, the IG showed a total normalization of the respiratory status, while in the CG, a small percentage of cases presented abnormal breath sounds and signs of respiratory effort, as chest retractions. Wheezing and chest retractions indicate an increase of ventilation effort, that in acute bronchiolitis may be related to inflammation, oedema and hyperproduction of mucus.20,27,28 Our results suggest that CP with PSE, RRC and PC was effective in removing secretions from the airway, decreasing bronchial obstruction and improving the respiratory status of children with mild-to-moderate bronchiolitis.
The reason for selecting these techniques was based on the pathophysiology of bronchiolitis in newborns and infants who have a very different anatomy and physiology in relation to older children or adults.10,20 In the four studies included in a recently published Cochrane review on CP in bronchiolitis, the use of PSE was reported to be associated with a reduction of the wheezing, respiratory work and discomfort of inpatients with bronchiolitis.8 Also, regarding RRC, there is some evidence of its effect in clearing the upper respiratory tract, and very encouraging results given that it is a non-pharmacological form of intervention without clinically relevant side-effects.23,28
The choice of an adequate CP technique is very important in regard to the safety and efficacy of intervention in bronchiolitis. Most guidelines worldwide discourage the use of classical CP (clapping, percussion or vibration technics) or acceleration of expiration flow in hospitalized children with bronchiolitis, as there is no evidence regarding its beneficial effect on reducing the length of hospital stay or on improving health status. Moreover, some of the techniques were associated with several adverse side-effects, such as atelectasis, vomiting and discomfort.29,30,31
Until today, only a few studies have focused on the use of more recent CP techniques in patients with bronchiolitis admitted to the hospital, leaving us with insufficient data to assess the efficacy of such techniques in improving clinical signs of upper and lower respiratory airways obstruction.11,12,20 Two studies, from 2011 and 2012, show a sustained reduction in the score used, over several days, suggesting that there is an accumulative effect of CP with the techniques of PSE and RRC.10,11 More recently, in 2020 a study compared the PSE and PC with high-frequency chest wall compression in outpatients with bronchiolitis.32 This mechanical device had the same positive results as the manual techniques. Both methods were able to reduce significantly the score and increase the airways clearance.32 Another study, carried out in Spain using the same techniques, obtained a reduction in hours of oxygen therapy during the period of hospitalization.10,11,12 In our study, the score was totally reduced after two weeks of treatment.
One of the major strengths of our study was the use of some of the most recent techniques of CP in children with mild-to-moderate bronchiolitis in an ambulatory setting, a situation in which CP might result in a faster recovery of the respiratory status. As stated, few studies have focused on the use of PSE and RRC in acute bronchiolitis and this study was the first to be conducted in a PED and continued in the ambulatory setting.8 The finding that CP is a relevant option in the management of mild and moderate cases of bronchiolitis in an outpatient setting is of utmost importance, given that it has shown to help avoid long recovery periods affecting both children and their families.28,33 Despite limited in scope, these findings confirm recent interest in these techniques, and surely warrant further studies and the collection of more data in support of a more robust understanding of the potential advantages and safety of these techniques.
As with any study, this study had expected limitations which we would like to address at this stage. The assessment of infants with bronchiolitis is difficult due to the clinical variability of the disease and there is a lack of evidence on the best tools to assess severity.2,28,34 Both physiotherapy techniques used in this study are highly specialized and need a well-trained physiotherapist to perform them. In our study, all the techniques were applied by the same physiotherapist, so results cannot be generalized to all practitioners. Also, there was a high rate of dropout in both arms of the study. Dropout in longitudinal randomized controlled trials is common and a potential source of bias.35 In both groups, treatment and assessment sessions non-attendance was the main reason for dropout, especially in the CG. In this group, this might be due to the fact that the parents/caregivers did not see any advantage in coming to the hospital only for regular clinical assessment. A sham intervention could have decreased the dropout rate in the CG, but ethical and psychological questions can be raised, and administering fake procedures is uncomfortable to professionals trained to perform interventions that they believe are useful for patients.36 The IG also showed a high rate of dropout, although the value was lower than in the CG. This leads us to question the number of sessions included in this study which may be too high and cumbersome. Future studies should take this into consideration and consider a lower number of sessions to address this issue.
Conclusion
In conclusion, our study showed that in an ambulatory setting, a CP intervention, based on passive prolonged slow expiration associated with rhinopharyngeal clearance and provoked cough, had a positive and significant impact on respiratory status of children under two years of age with mild-to-moderate bronchiolitis.
Acknowledgments
We would like to thank all the children and their parents/caregivers who participated in the study, all physicians and nurses from the Emergency Department involved in the study, Isabel Fonseca Ph.D. (Centro Hospitalar Universitário do Porto), and Sara Ramos Pinto Ph.D. (University of Leeds).
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this paper.
Funding/Support
There was no financial support for the study.
Author Contributions
Frederico Ramos Pinto conceptualized and designed the study, analyzed the data and drafted the initial manuscript and approved the final manuscript as submitted.
Ana Silva Alexandrino participated in the initial analyses, reviewed and revised the manuscript and approved the final manuscript as submitted.
Inês Azevedo and Liane Correia-Costa conceptualized and designed the study, supervised data collection, participated in and supervised data analyses, reviewed and revised the manuscript and approved the final manuscript as submitted.
References
- 1. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics 2017; 139(3) :e20163432. Crossref, Google Scholar
- 2. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134(5) :e1474–502. Crossref, Google Scholar
- 3. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr 2014; 40 :65. Crossref, Google Scholar
- 4. Bandeira T, et al. Norma I: Diagnóstico e Tratamento da Bronquiolite Aguda em Idade Pediátrica. Departamento da Qualidade na Saúde, Direção Geral da Saúde, http://nocs.pt/wp-content/uploads/2015/11/Diagn%C3%B3stico-e-Tratamento-da-Bronquiolite-Aguda-em-Idade-Pedi%C3%A1trica.pdf, 2012. Google Scholar
- 5. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child 2016; 101(2) :140–6. Crossref, Google Scholar
- 6. Trends in hospitalization for acute bronchiolitis in Portugal: 2000-2015. Pulmonology 2019; 25(3) :154–61. Crossref, Google Scholar
- 7. Management of bronchiolitis in community hospitals in Ontario: A multicentre cohort study. CJEM 2016; 18(6) :443–52. Crossref, Google Scholar
- 8. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev 2016; 2(2):CD004873. Google Scholar
- 9. . Effects of the use of respiratory physiotherapy in children admitted with acute viral bronchiolitis. Arch Pediatr 2018; 25(6) :394–8. Crossref, Google Scholar
- 10. Evaluation of an alternative chest physiotherapy method in infants with respiratory syncytial virus bronchiolitis. Respir Care 2011; 56(7) :989–94. Crossref, Google Scholar
- 11. Chest physical therapy is effective in reducing the clinical score in bronchiolitis: Randomized controlled trial. Rev Bras Físioter 2012; 16(3) :241–7. Crossref, Google Scholar
- 12. [Chest physiotherapy and bronchiolitis in the hospitalised infant. Double-blind clinical trial]. An Pediatr (Barc) 2012; 77(1) :5–11. Google Scholar
- 13. Comparative analysis of the effects of two chest physical therapy interventions in patients with bronchiolitis during hospitalization period. Einstein (Sao Paulo) 2014; 12(4) :452–8. Crossref, Google Scholar
- 14. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63(8) :834–40. Crossref, Google Scholar
- 15. . Properties of permuted-block randomization in clinical trials. Control Clin Trials 1988; 9(4) :327–44. Crossref, Google Scholar
- 16. Nebulised racemic adrenaline in the treatment of acute bronchiolitis in infants and toddlers. Arch Dis Child 1993; 69(6) :650–4. Crossref, Google Scholar
- 17. . Reliability and validity of the respiratory score in the assessment of acute bronchiolitis. Malays J Med Sci 2004; 11(2) :34–40. Google Scholar
- 18. . Comparison of Kristjansson Respiratory Score and Wang Respiratory Score in infants with bronchiolitis in a hospital emergency department. Hong Kong Physiother J 2020; 40 :145–53. Link, Google Scholar
- 19. Diagnosis and testing in bronchiolitis: A systematic review. Arch Pediatr Adolesc Med 2004; 158(2) :119–26. Crossref, Google Scholar
- 20. [Respiratory physiotherapy in acute viral bronchiolitis in the newborn. Pro/con arguments]. Rev Mal Respir 2018; 35(4) :403–15. Crossref, Google Scholar
- 21. Postiaux G. Fisioterapia Respiratória Pediátrica — o Tratamento Guiado por Ausculta Pulmonar. 2nd ed. Porto Alegre: Artmed, 2004. Google Scholar
- 22. . Rhinopharyngeal retrograde clearance induces less respiratory effort and fewer adverse effects in comparison with nasopharyngeal aspiration in infants with acute viral bronchiolitis. Respir Care 2016; 61(12) :1613–9. Crossref, Google Scholar
- 23. Caregivers’ education vs rhinopharyngeal clearance in children with upper respiratory infections: Impact on children’s health outcomes. Eur J Pediatr 2017; 176(10) :1375–83. Crossref, Google Scholar
- 24. . Using Multivariate Statistics. 6th ed. Boston, MA: Pearson Education, 2012. Google Scholar
- 25. . Avaliação da normalidade dos dados em estudos clínicos e experimentais. J Vasc Bras 2017; 16(2) :88–91. Crossref, Google Scholar
- 26. Marôco J. Análise Estatistica com o PASW Statistics. Report Number: 07-2010, 2010. Google Scholar
- 27. . Chest physical therapy in acute viral bronchiolitis: An updated review. Respir Care 2013; 58(9) :1541–5. Crossref, Google Scholar
- 28. . Current concepts in the evaluation and management of bronchiolitis. Infect Dis Clin North Am 2018; 32(1) :35–45. Crossref, Google Scholar
- 29. [Indications of conventional chest physiotherapy in acute bronchiolitis]. Medicina (B Aires) 2004; 64(3) :198–200. Google Scholar
- 30. Effectiveness of chest physiotherapy in infants hospitalized with acute bronchiolitis: A multicenter, randomized, controlled trial. PLoS Med 2010; 7(9): e1000345. Crossref, Google Scholar
- 31. Chest physiotherapy using passive expiratory techniques does not reduce bronchiolitis severity: A randomised controlled trial. Eur J Pediatr 2012; 171(3) :457–62. Crossref, Google Scholar
- 32. Immediate effects and safety of high-frequency chest wall compression compared to airway clearance techniques in non-hospitalized infants with acute viral bronchiolitis. Respir Care 2020; 66(3) :425–33. Crossref, Google Scholar
- 33. Practice variation in acute bronchiolitis: A pediatric emergency research networks study. Pediatrics 2017; 140(6):e20170842. Crossref, Google Scholar
- 34. . A systematic review of the psychometric properties of bronchiolitis assessment tools. J Adv Nurs 2017; 73(2) :286–301. Crossref, Google Scholar
- 35. Differential dropout and bias in randomised controlled trials: When it matters and when it may not. BMJ 2013; 346 :e8668. Crossref, Google Scholar
- 36. . Sham procedures and the ethics of clinical trials. J R Soc Med 2004; 97(12) :576–8. Crossref, Google Scholar


