Synthesis, in vitro inhibition effect of novel phthalocyanine complexes as carbonic anhydrase and paraoxonase enzyme inhibitors
Abstract
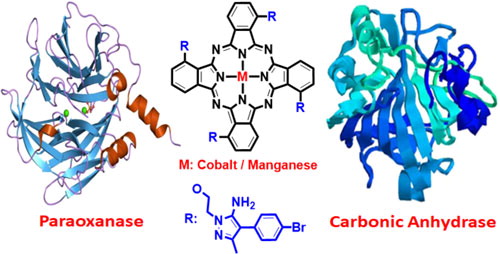
The preparation and assessment of carbonic anhydrase and paraoxonase enzyme inhibition properties of 3-(2-(5-amino-4-(4-bromophenyl)-3-methyl-1H-pyrazol-1-yl)ethoxy)phthalonitrile (2) and its nitrogen-containing non-peripheral phthalocyanine derivatives (3 and 4) are reported for the first time. The new phthalonitrile and its phthalocyanine derivatives have been elucidated by FT-IR spectroscopy, 1H-NMR, C-NMR, mass and UV-vis spectroscopy. The results demonstrated that all synthesized compounds moderately inhibited carbonic anhydrase and paraoxonase enzymes. Among the compounds, the most active ones were found to be compound 4 for PON (Ki : 0.14 M), compound 3 for hCA I (Ki : 22.52 M) and compound 1 for hCA II (Ki : 13.62 M).
Handbook of Porphyrin Science now available in 46 volumes


