Rapid determination of tannin in Danshen and Guanxinning injections using UV spectrophotometry for quality control
Abstract
A technique for the determination of tannin content in traditional Chinese medicine injections (TCMI) was developed based on ultraviolet (UV) spectroscopy. Chemometrics were used to construct a mathematical model of absorption spectrum and tannin reference content of Danshen and Guanxinning injections, and the model was verified and applied. The results showed that the established UV-based spectral partial least squares regression (PLS) tannin content model performed well with a correlation coefficient (rr) of 0.952, root mean square error of calibration (RMSEC) of 0.476μμg/ml, root mean square error of validation (RMSEV) of 1.171μμg/ml, and root mean square error of prediction (RMSEP) of 0.465μμg/ml. Pattern recognition models using linear discriminant analysis (LDA) and kk nearest neighbor (kk-NN) classifiers based on UV spectrum could successfully classify different types of injections and different manufacturers. The established method to measure tannin content based on UV spectroscopy is simple, rapid and reliable and provides technical support for quality control of tannin in Chinese medicine injections.
1. Introduction
Tannins, also named as Ning or tannic acid, are a class of water-soluble high molecular polyphenols1 that are widely found in traditional Chinese medicines (TCM), and have the ability to precipitate proteins. The presence of tannins in traditional Chinese medicine injection (TCMI) not only affects the stability and clarity of the preparation, but also may cause a series of serious physiological reactions such as local tissue tumor, necrosis, and subcutaneous hemorrhage.2 Therefore, almost all TCMI production processes contain a step of removing tannins, and tannin examination is also a special inspection item for TCMIs.
There are many methods for determining the content of tannins, among which the most commonly used methods are the skin powder method, casein method, electrochemical analysis and high-performance liquid chromatography (HPLC) method.1,3,4,5 The current edition (2015) of the Chinese Pharmacopoeia6 lists phosphomolybdate as a chromogenic reagent, gallic acid as a reference substance, and casein as an adsorbent for determination of tannin content. This method is widely used to determine tannin content of TCM.7,8 Although the selectivity of the casein method is superior to the classical skin powder method, the operation is cumbersome and time-consuming. Preparation and validation of the photophosphorus molybdenum tungstate reagent take 10h, which is problematic in coping with large-scale Chinese medicine preparation and production and the requirements for analysis and monitoring of tannin content.
Given the need to analyze large batches of samples during production, correspondingly higher requirements are imposed on the rapidity and simplicity of the test methods. Therefore, spectral technologies represented by fluorescence and near-infrared (NIR) spectroscopy have received increased attention.9,10,11 For example, Hung et al.12 used fluorescence flow-injection analysis to determine the tannin and total amino acid content in tea as a method to rate the quality of fermented tea. Xing et al.13 proposed a method that based on the first-order octave interval of the aromatic-carbon carbon-hydrogen bond (5430–5839cm−1−1) of NIR spectroscopy can accurately reflect the structural characteristics of the tannin component. The method with high correlation coefficient and low prediction error could be used for rapid determination of tannins during the alcohol precipitation process of Danshen injection. Carbon dots, which are a new type of carbon nanomaterial, have good fluorescence properties and biocompatibility, and their applications have also been widely examined. Currently, advanced sensors such as fluorescent carbon dots and photoelectrochemicals are mainly used for the detection of tannin. Gaber14 used carbon nano-dots to directly measure the tannin content in red and white wine samples. The results show that the method has high sensitivity, strong selectivity, and a wide range of linear response. Shi15 offered the same carbon dots technology for detection of tannin in the water sample. The method had the wide linear range of 0.2–10.0μμmol/L, and low detection limit of 9.0nmol/L. Fernanda16 developed a self-powered photoelectrochemical sensor for the determination of tannic acid based on a tannic acid-sensitized TiO2 (TA/TiO2/FTO) as photoanode and Cu2O/ZnO/FTO photocathode to water reduction. The response tannic acid range was from 1 to 500μμmol/L. These methods provide new ideas for the rapid detection of tannin content in TCMIs.
In this paper, the Danshen and Guanxinning injections, which are single-variety TCMIs that are currently top selling in China, were taken as research examples. Danshen injection is prepared from the aqueous extract of Salviae Miltiorrhizae Radix et Rhizoma (Danshen), and the medicinal carrier. Guanxinning injection is made from the aqueous extract and alcohol precipitation of Danshen and Chuanxiong Rhizome (Chuanxiong). Both of these injections promote blood circulation and prevent blood stasis, and are commonly used in the treatment of coronary heart disease, angina pectoris, and myocardial infarction.17,18,19 Given that tannin is a polyphenolic compound, they show strong absorption in the ultraviolet (UV) region such that characteristic information can be derived from the UV absorption spectrum. Thus, a method is proposed for the rapid determination of tannin content in TCMIs by using UV absorption spectra. It provides a method and idea for the quality control of tannins in TCMI.
2. Experiment and Methods
2.1. Materials
Gallic acid reference product was purchased from Shanghai Ronghe Pharmaceutical Technology Co., Ltd. (batch number 130007); casein was purchased from Zhejiang Shuanglin Chemical (batch number 20120112); sodium hydroxide was purchased from Zhejiang Zhongxing Chemical (batch number 20110120). The anhydrous sodium carbonate and Folin test solutions were purchased from Sinopharm (batch numbers 20120228 and 20130922, respectively).
A total of 51 Danshen (31) and Guanxinning (20) injections were purchased in the market. Among them, 41 samples for a calibration model. The 26 Danshen injections were produced by three different manufacturers: manufacturer A (10), manufacturer B (9), and manufacturer C (7). The 15 Guanxinning injections were produced by three different manufacturers: manufacturer D (5), manufacturer E (7), and manufacturer F (3). Remaining 10 samples were used for external verification.
2.2. Instruments
Experiments utilized the following instrumentation: AR224CN analytical balance (Ohaus Instruments, Shanghai, China); TU-1901 dual beam UV-Visible spectrophotometer (Beijing PuXi General Instrument, Beijing, China).
2.3. Methods
2.3.1. Tannin reference value determination
The test solution was prepared by diluting 2ml of the injection solution in a brown 100ml volumetric flask with water.
The tannin content of Danshen and Guanxinning injections were determined according to the modified casein method.1 The method as described in the 2015 Chinese Pharmacopoeia was optimized and improved for the experimental conditions and reagents for the determination of tannin content. The main modifications included replacement of 29% Na2CO3 solution with a mixture of 14% Na2CO3 solution and 0.03M NaOH solution, and replacement of the phosphomolybdate reagent with a commercially available Folin reagent.
2.3.2. Acquisition of UV spectrum
UV spectra were collected using a 1cm quartz cuvette with a wavelength range of 200–480nm, a wavelength interval of 1.0nm, and a medium scan speed. Deionized water was used as the blank, and the spectrum of each sample was recorded as the average of three scans.
2.3.3. Classification modeling based on UV spectrum
To identify different injections and different manufacturers of the same injection based on UV spectrum, two classical pattern recognition techniques, i.e., linear discriminant analysis (LDA) and kk nearest neighbor (kk-NN) were used. The mathematical algorithm was performed with in-house programs in MATLAB R2015a (Mathworks Inc.).
LDA hypothesizes that the distribution of the input variable (UV wavelength) is multivariate normal and there is no significant difference in the covariance matrix of each class from each other. The Mahalanobis distances of a tested sample to the centroids of all classes are calculated, and they are assigned to the class with the shortest distance.20 In this method, a linear function is normally used to delimit between classes.
As a nonparametric classification method, kk-NN does not formulate a hypothesis on the distribution of the input variables.21 The classification is rather performed using a training dataset that contains the input variable and target variable (i.e., class information). After a model is developed on the training dataset, it is challenged by the tested samples. Once the kk nearest samples are defined, the tested sample is classified to the class where the majority of the kk nearest sample belongs to.
A leave-one-out (LOO) cross-validation approach is used to evaluate the performance of the classifier, in which one out of every 41 samples is excluded, and its classification is predicted by the model established from the remaining 40 samples as training data. The process is repeated until all samples in the training set are left out once, and the prediction results of the tested samples are then compared with the known results.
2.3.4. Establishment and verification of multivariate calibration model
The tannin content determined by the modified casein method was used as a reference value, and a regression model between the reference value and the UV spectrum was established using a multivariate correction method. Multivariate correction methods include multiple linear regression (MLR), principal component regression (PCR), and partial least squares regression (PLS). Application of MLR is effective when the spectral signal is linearly related to the concentration of the analyte, there is no interaction between the analytes, and the spectral noise is small. However, this method can only process a certain number of wavelength signals, and thus loses some useful information in the spectrum. In addition, if there is severe collinearity in the spectral data, the accuracy will be greatly reduced when using MLR for prediction. The PCR method and the PLS regression can make up for the shortcomings of the MLR method.22 On the one hand, the data can be fully utilized by using the overall measured data. However, the noise filtering can be performed by principal component selection, effectively solving the collinear problem, and the complex system. The analysis and testing of substances is also very suitable. The composition of TCM is complex. For this reason, the mathematical model between spectral and tannin content was established by PCR and PLS,23 respectively. The calibration set consists of 281 xx variables (UV spectrum) and 41 yy variables (tannin content).
External verification of the model is also required before the multivariate calibration model is applied. Ten injection samples with known tannin content (the tannin content needs to fall within the calibration set tannin content range), the UV spectra were scanned according to the same acquisition conditions, and the spectral eigenvalues were input into the established calibration. In the model, the sample tannin content is calculated and compared with the reference value.
The evaluation parameters of the calibration model and the verification model include correlation coefficient (rr), root mean square error of calibration (MSEC), root mean square error of validation (RMSEV), and root mean square error of prediction (RMSEP). The rr value between the UV prediction and the measured value was calculated as follows :
The RMSE was calculated with the following expression :
Spectroscopic analysis and a quantitative model for rapid determination of tannin content based on UV spectra were performed using Unscrambler 9.7 (CAMO, Magnolia, TX, USA).
3. Results and Discussion
3.1. Methodological study on modified casein method
The reference value of the tannin content is determined by the modified casein method reported by Zhang et al.1 However, this method is currently only used for the determination of tannin content in Danshen injection, and not in Guanxinning injection. Before using this method to determine the tannin content of the injections, a methodological investigation was carried out.
3.1.1. Color reaction time
For the total phenol stability test, six samples of the same batch of Guanxining injection were diluted, and the absorbance measured at 20, 30, 40, 50 and 60min. The results are shown in Table 1.
| Color reaction time (min) | A (n=6) | RSD (%) |
|---|---|---|
| 20 | 0.922 | 0.539 |
| 30 | 0.974 | 0.459 |
| 40 | 1.019 | 0.375 |
| 50 | 1.050 | 0.334 |
| 60 | 1.075 | 0.409 |
For the nonadsorbed polyphenol stability test, accurately measure six of diluted sample 25.0 mL, and the absorbance measured at 20, 30, 40, 50 and 60min, respectively. The results are shown in Table 2.
| Color reaction time (min) | A (n=6) | RSD (%) |
|---|---|---|
| 20 | 0.546 | 1.660 |
| 30 | 0.598 | 1.122 |
| 40 | 0.633 | 0.957 |
| 50 | 0.655 | 1.598 |
| 60 | 0.678 | 1.085 |
In Tables 1 and 2, the RSD values were lowest for the 30–40min interval. The color reactions were more stable during this time, and there was no significant difference in absorbance after 30min. Therefore, the color reaction time was set to 30min.
3.1.2. Linear investigation
Aliquots of the reference solution (0.5, 1.0, 2.0, 3.0, 4.0, 5.0ml) were accurately measured and the absorbance was measured by the modified casein method. Plotting of concentration–absorbance data gave the regression equation of y=0.0687x−0.0252, (r=0.9992). The absorbance and concentration of the tannin showed a good linear relationship in the range of 1.0–10.0μg/ml.
3.1.3. Reproducibility
Six samples of the same batch of injection were taken and measured in parallel with the same method. The results showed that the sample had the same tannin content, the average concentration was 1.424μg/mL, and the RSD was 0.0450%. The method has good reproducibility.
3.1.4. Precision
The same injection sample was analyzed three times at different time points on the same day. The measured sample had the same tannin content, the average concentration was 1.448μg/mL, and the RSD was 0.7678%, indicating that intra-day precision was good.
3.1.5. Stability
The same injection sample was analyzed at different time points of 0, 4, 8, 12, 16, 24h. The sample was found to have the same tannin content, with an average concentration of 1.456μg/mL and an RSD of 1.386%. The sample and method were deemed stable over 24h.
3.2. UV spectroscopy
A total of 41 injection samples (including 26 Danshen injections and 15 Guanxinning injections) were collected, and the original UV spectra are shown in Fig. 1. From the tannin reference gallic acid and a batch of injection samples (Fig. 2), in addition to the terminal absorption at 200nm, the tannin has a maximum absorption peak at 250nm, and the injection has a maximum at 280nm. The absorption peaks provide a reference for the selection of the model to establish the band. PCA analysis was performed on 41 injection samples, where PC1 explained 99% of the total variance. The scores of the first two PCs (see Fig. 3) well divided the sample into two different injection types. The dispersion of Guanxinning injection samples was larger than that of Danshen injection samples.
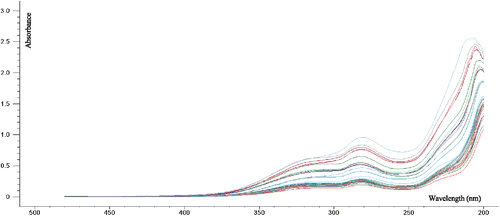
Fig. 1. Raw UV spectrum of 41 injection samples.
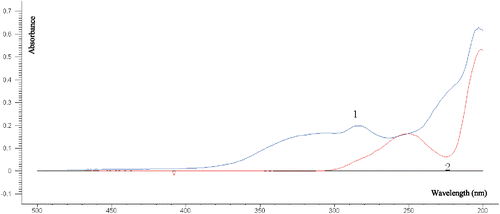
Fig. 2. Comparison of UV spectra of gallic acid reference and a Danshen injection (1, Danshen injection; 2, gallic acid).
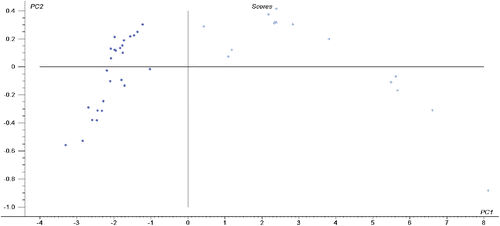
Fig. 3. PCA score of the injection sample UV spectrum (solid circle, Danshen injection; hollow circle, Guanxinning injection).
Distinguishing different injections and different manufacturer of the same injection is of important concern to both health authorities and the public. In this work, we further evaluated two classifiers, LDA and k-NN, as potential tools to determine the types of injections and manufacturers based on UV spectra. The classification results summarized in Table 3 showed that different types of injections and manufacturers could be identified with 100% accuracy by either LDA or k-NN based on UV spectroscopy. It indicated that UV spectroscopy contains sufficient discriminatory power to distinguish the injection type and manufacturers and are thus applicable for monitoring the TCM products.
| Injections | Total | Correct | Manufacturers | Total | Correct | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Classifiers | Nr∕NDS | Nr∕NGXN | Nr∕N0 | % | Nr∕NA | Nr∕NB | Nr∕NC | Nr∕ND | Nr∕NE | Nr∕NF | Nr∕N0 | % |
| LDA | 26/26 | 15/15 | 41/41 | 100 | 10/10 | 9/9 | 7/7 | 5/5 | 7/7 | 3/3 | 41/41 | 100 |
| k-NN | 26/26 | 15/15 | 41/41 | 100 | 10/10 | 9/9 | 7/7 | 5/5 | 7/7 | 3/3 | 41/41 | 100 |
3.3. Establishment of a multivariate correction model
3.3.1. Investigation of spectral pretreatment methods for PLS regression model
The mathematical correction model between the UV spectrum and tannin content was established by PLS regression. The calibration set samples consisted of 41 x variables (UV spectra) and y variables (tannin content). The two most commonly used validation methods are cross-validation and test set validation. In this study, LOO cross-validation was applied as an internal validation and an unseen set of samples, not included in the calibration model, were used for external validation. The model was optimized by examining the effects of various spectral preprocessing methods such as Savitzky-Golay (SG) smooth, MSC, SNV, SG+1stD, and baseline. The results were shown in Table 4 with r, RMSEC, and RMSEV being performance indicators of the model. The best UV spectral pretreatment method was the SG+1stD method. The optimal number of PCs was 5.
| Pretreatment method | PCs | r | RMSEC (μg/mL) | RMSEV (μg/mL) |
|---|---|---|---|---|
| Raw spectrum | 6 | 0.844 | 0.855 | 1.305 |
| SG smooth | 6 | 0.832 | 0.887 | 1.420 |
| MSC | 4 | 0.703 | 1.180 | 1.522 |
| SNV | 4 | 0.703 | 1.180 | 1.611 |
| Baseline | 5 | 0.782 | 1.011 | 1.499 |
| SG+1stD | 5 | 0.952 | 0.476 | 1.171 |
3.3.2. Selection of PLS model band
Based on the selection of the SG+1stD method as the optimal UV spectrum pretreatment, PLS combined with the full band and different bands to establish the calibration model of each index, using r, RMSEC, and RMSEV as the performance indicators. Table 5 shows the analysis result of the model. When the model selects the full band, the built model has the best performance.
| Pretreatment method | PCs | r | RMSEC (μg/mL) | RMSEV (μg/mL) |
|---|---|---|---|---|
| 200–480 | 5 | 0.952 | 0.476 | 1.171 |
| 200–280 | 8 | 0.849 | 0.840 | 1.919 |
| 250–280 | 5 | 0.806 | 0.954 | 1.865 |
| 250–480 | 4 | 0.871 | 0.778 | 1.155 |
| 280–480 | 3 | 0.810 | 0.943 | 1.071 |
It can be seen from Tables 4 and 5 that the model performance is relatively optimal when the full band of 200–480nm is selected for the PLS model: r=0.952, RMSEC = 0.476μg/mL and RMSEV = 1.171μg/mL.
The tannin content PLS regression model was established according to the modeling band selected above. Figure 4 is a correlation diagram between the predicted value of the tannin content model established by the PLS method and the reference value. It can be seen from Fig. 4 that the correlation between the predicted value of the PLS model and the reference value is good, and the model performance is excellent.
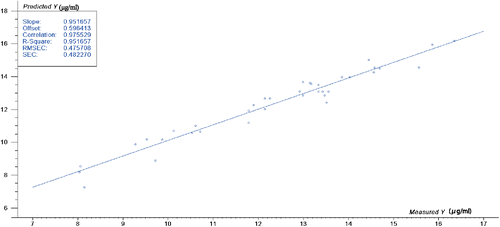
Fig. 4. Predicted and measured values of tannin in injection samples.
We performed PCR modeling in the same way. SG smoothing was selected as the preprocessing method, and the model established in the 280–480nm band was the best: r=0.911, RMSEC = 0.645μg/mL, and RMSEV = 1.015μg/mL. In comparing the performance of the two regression models, the PLS method was found to establish a better regression model for tannin content.
3.4. Calibration model verification
The established PLS regression model for tannin content was externally verified and the results were shown in Table 6. The RMSEP value was 0.465μg/mL, which was an acceptable prediction error.
| No. | Reference (μg/mL) | Predictive (μg/mL) | RMSEP (μg/mL) |
|---|---|---|---|
| 1 | 9.284 | 9.866 | 0.465 |
| 2 | 8.056 | 8.532 | |
| 3 | 9.716 | 8.979 | |
| 4 | 11.900 | 12.259 | |
| 5 | 10.717 | 10.651 | |
| 6 | 10.603 | 11.007 | |
| 7 | 10.125 | 10.689 | |
| 8 | 9.534 | 10.176 | |
| 9 | 13.855 | 13.965 | |
| 10 | 12.150 | 12.018 |
4. Conclusion
In this study, a method for the rapid determination of tannin in Danshen and Guanxinning injections was established based on features of the UV spectrum. The results showed that the performance of the PLS model was superior to that of the PCR model. Therefore, using the PLS model, the SG+1stD pretreatment method was used to select the full UV band of 200–480nm, of which r is 0.952, RMSEC is 0.476μg/ml, RMSEV is 1.171μg/ml. The predicted value of the sample tannin content was close to the true value, with RMSEP of 0.465μg/ml. In addition, different types of injections and manufacturers could be identified with 100% accuracy by either LDA or k-NN based on UV spectroscopy. Because the method of measuring the UV spectrum is simple in operation, uses common instrumentation, and is accurate and fast, its requirements for manpower and material resources are modest. It is suitable for the analysis of tannin content in large numbers of samples in production, and provides a reliable method for the quality control of traditional Chinese medicine injections.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was supported by the State Administration of Traditional Chinese Medicine of Zhejiang Province Project (Grant No. 2015ZQ022), Zhejiang TCM Health Science and Technology Project (Grant No. 2015KYB110), and Zhejiang Provincial Natural Science Foundation of China (Grant No. LY17B020002).