Power spectral density-based nearinfrared sub-band detection for noninvasive blood glucose prediction in both in-vitro and in-vivo studies
Abstract
Diabetes is a widespread and serious disease and noninvasive measurement has been in high demand. To address this problem, a power spectral density-based method was offered for determining glucose sensitive sub-bands in the nearinfrared (NIR) spectrum. The experiments were conducted using phantoms of different optical properties in-vitro conditions. The optical bands 1200–1300nm and 2100–2200nm were found feasible for measuring blood glucose. After that, a photoplethysmography (PPG)-based low cost and portable optical system was designed. It has six different NIR wavelength LEDs for illumination and an InGaAs photodiode for detection. Optical density values were calculated through the system and used as independent variables for multiple linear regression analysis. The results of blood glucose levels for 24 known healthy subjects showed that the optical system prediction was nearly 80% in the A zone and 20% in the B zone according to the Clarke Error Grid analysis. It was shown that a promising easy-use, continuous, and compact optical system had been designed.
1. Introduction
Noninvasive blood glucose measurements have been studied for four decades. It is still a popular and worthwhile research topic due to the fact that the number of diabetic people has increased dramatically in recent years. The latest global report on diabetes by the World Health Organization points out that one out of eleven people is diabetic worldwide.1 It is suggested by health institutions that diabetic patients should check their blood glucose levels 3 or 4 times daily to control their glycosylated hemoglobin levels. This continuous monitoring is crucial for a quality life standard. Therefore, noninvasive blood glucose measurement remains a hot topic. The number of patents which contain noninvasive blood glucose keywords is shown in Fig. 1. As it can be seen in the graph, the attention of researchers has been growing significantly year by year since the 1970s. Yet, there is no NIH approved noninvasive blood glucose measurement device or method.2,3 Some of the noninvasive blood glucose measurement study summaries are described in what follows.
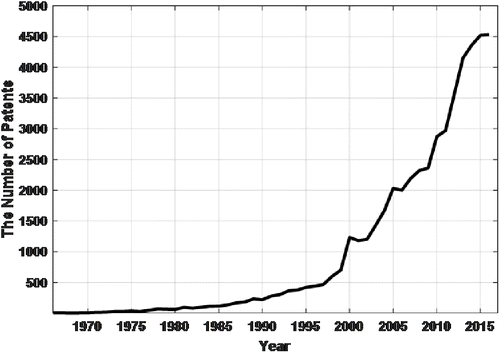
Fig. 1. The number of patents that involve noninvasive blood glucose keywords by year.
Shen et al. (2003) researched blood glucose level determination using the Fourier Transform Infra-red Spectroscopy (FTIRS) technique.4 Their study focused on the mid-infrared region between 1200–950cm−1 (8333–10,526nm). The transmission based measurements were taken from 28 subjects. In their analysis, they claimed that the difference of the second derivatives of 9149 and 9242 nm spectrum values were correlated with glucose. They claimed that the glucose concentration in the blood could be defined by using two wavelengths. They analyzed the blood samples which were taken from the subjects and put them into the cuvettes. Actually, there are two main drawbacks to studying the mid-infrared spectral region. One of them is that the absorption of the water is too high. Furthermore, the glucose absorption was dominated by the water. The second drawback is that the light penetration of the tissue is quite limited in this spectral region.2,5 In addition, working in this spectral region, neither the light source nor the detector is cost effective. Moreover, their study was proposed as an ex-vivo technique.
Zhu et al. (2013) worked on an ultrasound modulated optical method for noninvasive blood glucose measurement.6 Their starting point was the fact that ultrasound waves scatter less than light waves. Additionally, the glucose and the scattering are inversely proportional.7,8 Consequently, the relation between scattering and glucose concentrations was investigated. Briefly, that means when the glucose concentration increases, the scattering coefficient of the medium will decrease. They claimed that a high correlation was observed between modulation depth which was the division of the peak to peak value of the oscillated signal to the average value, and glucose concentrations within the liquid phantoms. However, there were some serious problems during the in-vivo experiments. As mentioned in their paper, the small temperature changes due to the modulation effect would increase the glucose concentration prediction errors. The physical variabilities, such as breathing, small motion artifacts, etc. would affect results adversely. Moreover, other blood components interfere with the scattering, too; it would cause a growing prediction error. Therefore, the study was limited to only in-vitro conditions.
Another study was done by Ozana et al. (2014) who developed a noncontact sensor for glucose detection.9 The tissue was illuminated by a 543nm He–Ne laser and the surface of the tissue images were captured by a high speed (545 fps) CMOS camera. The variances of random laser speckle patterns were investigated. Each speckle pattern of the sequential image frames was studied for the correlation with the tissue. In addition, a magnet was used for applying a magnetic field on speckle variances. The main purpose of using the magnet was that only glucose molecules interact with magnetism.9 The reason is that the Verdet constant of the glucose is higher than the other blood analytes. The glucose concentration was determined via wrist images from four subjects. The authors mentioned that there was a correlation between the speckle patterns and glucose levels. However, when the results were investigated, the prediction error and standard deviations were too high. Also, their method needed individual calibration so that it was impractical in real life. Moreover, the method was also very sensitive to motion artifacts.
Another popular noninvasive blood glucose measurement technique is Optical Coherence Tomography (OCT), which is based on a low coherence length light source and an interferometry system.10,11 In OCT, the light source is split into two arms where one of them is the reference and the other one is the sample arm. There is a scanning mirror which oscillates at a certain frequency, on the reference arm. The back-reflected beams are interfered and measured with a photodetector. The amount of delay from the back-reflected beams through the sample arm carries information about the medium.10,11 Though it is very promising and a popular optical measurement system, very small movements or motions cause large errors. Furthermore, it is a high cost, bulky system and is affected by temperature changes, all of which are disadvantages.2,12
A different optical blood glucose measurement technique is Photoacoustic Spectroscopy (PS). It is based on the energy conversion principle. When the photons that are excited by a very fast pulse light source, i.e., femtosecond, illuminate the sample, a vibration comes into existence. The propagated sound waves due to the vibrations are detected by piezo sensors. Therefore, the light energy is converted to mechanical energy; after that, it is transformed to sound energy. This is called photoacoustic. The differences between glucose concentrations are shown to be observable through photoacoustic signals. However, the main difficulty is that each different sample reacts to different wavelengths. Therefore, it is hard to use a general blood glucose measurement technique.9,13
There are some studies which contributed in part to our studies, based on photoplethysmography (PPG). In 2006, Yamakoshi and Yamakoshi proposed a noninvasive blood glucose measurement method which they called pulse glucometry.14 In the method, PPG signals were recorded by an ultrafast 900–1700nm NIR spectrophotometer with 1800 spectrum/s rate.14,15 Derivative information was extracted through the spectrums, and then an artificial neural network (ANN) was used to predict blood glucose levels. While ANN was trained and tested, 27 healthy subjects were used and 603 pieces of data were obtained in total. The main negative aspect of the system was prohibitively expensive. Specifically the spectrophotometer was one of the high-tech products used and not easily affordable for many people and clinics.
In our study, we aimed for measuring noninvasive blood glucose levels by following a 3C (cost, convenient, comfort) optical system. The system is capable of eliminating large scattering effects and other disturbances such as other blood components. We offered a power spectral density method to represent glucose absorption band in-vitro conditions. Then an optical system was designed to monitor and predict blood glucose level. The details of the method and the designed system are given in the next sections.
2. Material and Methods
This study can be split into two sections. The first part focuses on in-vitro studies which aimed to determine glucose representative sub-bands in the NIR spectrum. The second part concentrates on in-vivo studies predicting the blood glucose level using a low cost and portable optical self-designed device.
2.1. Determining of power spectral density based glucose representative optical bands in the near infra-red region
The flow chart of the proposed algorithm to determine glucose sensitive optical bands in the NIR spectrum is given in Fig. 2.
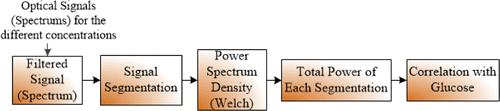
Fig. 2. The proposed power spectral density based algorithm flow chart.
As you can see, the transmitted spectrums of the different glucose concentrations in liquid phantoms were measured. The spectrums were obtained with an NIR spectrophotometer (NIRQuest512, Ocean Optics, USA) that covers the range 900–2500nm. In the first step, the spectrums were pre-processed by a 7 point moving average filter. Then a baseline removing filter was applied because of small fluctuations of the spectrophotometer. The next step was the segmentation of the spectrum to its sub-bands. The filtered spectrums were segmented and 50% overlapped with a Hanning window. The length of each segment was set at about 100 nm due to the size of the spectrophotometer detector array. In addition, shorter segment lengths showed almost no noticeable difference. After that, the total power for each segment was calculated by using the Welch power spectral density estimation method. The aim of the study was to investigate the sub-band power and glucose relationship. The correlations between all different glucose concentrations against the measured power values were calculated for all sub-bands. The representative sub-band of the glucose was analyzed according to their correlation coefficients and p values.
Three tissue phantoms which had different scattering coefficients were prepared by using an intralipid solution (ClinOleic 20%) for the scatter and black Indian ink (Higgins) for the absorber agents.16,17,18,19,20 It is known that the glucose absorption is a result of scattering.21,22 The phantoms whose reduced scattering coefficients were μ1s=5.5μ1s=11 and μ1s=22cm−1, and whose absorption coefficient was μa=0.15cm−1 were prepared. After that, the glucose was added to each phantom medium from 0–1000mg/dl in 100mg/dl increments. Then all the spectrums were measured and recorded for the glucose representative sub-band analysis.
2.1.1. PPG Based blood glucose measurement system design
In the first step of the study that was explained above, the glucose representative sub-bands were evaluated under in-vitro conditions. According to obtained results, a photoplethysmography (PPG) based optical system was designed. PPG is a light absorption based blood pressure monitoring technique. It is still a very popular method because of ease of use, low cost and being noninvasive.23,24,25 The detailed information for those who are curious about PPG can be found in Ref. 23.
During regular PPG measurements, errors may be caused due to physical differences in the subjects which render the PPG inadequate to derive the components from the blood.26,27 The absorption values of skin, muscle, bone, fat, etc. should be eliminated. They have permanent absorption values and should be excluded from the blood absorption value during measurement. To eliminate or minimize these effects, the PPG based dynamic spectrum has been proposed.14,26,27,28 In this method, a finger or any kind of tissue on a human can be divided into three layers. These are arteries, veins and other tissues than the blood such as bone, skin, nails, etc.14,26,27 The artery layer can also be split into two sections. One of them is static, and the other part is pulsatile. This is shown in Fig. 3. While calculating the dynamic spectrum, the pulsatile part that represents the blood pressure alterations over time is important.
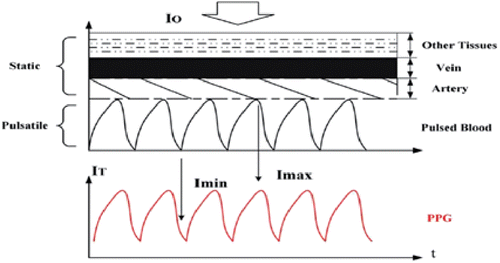
Fig. 3. Multilayer structure of the tissue.28
When I0, which is the incident beam, illuminates the fingertip perpendicularly during transmittance based measurement, the heart beat and the blood pressure in the capillaries cause maximum and minimum light intensity changes. Imax and Imin are detected by the photodiode on the other side of the fingertip or tissue. It has been declared that transmission based measurements have better glucose detection than reflectance.8 These Imax and Imin intensities indicate the systolic and diastolic situations of blood pressure, respectively. The logarithmic difference of these two intensities gives the optical absorption difference ΔOD,
The self-designed optical measurement system was an alternative to similar measurement systems. However, the important improvement is that the system had just one InGaAs photodiode (SD039-151-011, API, USA) as a detector instead of expensive spectrometer or detector arrays. In addition, the self-designed system was using six different NIR wavelengths LED light sources which were 1070, 1200, 1300, 1450, 1550 and 1650nm (LED1070L, LED1200L LED1300L, LED1450L, LED1550L, LED1650L, Thorlabs, USA). The wavelengths were selected according to our in-vitro studies mentioned above. The self-designed system block diagram is given in Fig. 4.
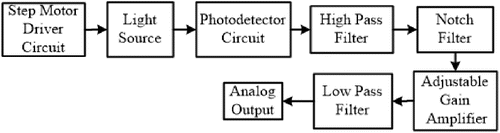
Fig. 4. The self-designed optical measurement system block diagram.
The system has a step motor which controls the LEDs position. The step motor rotates each LED, which were placed equidistance apart forming a circle (Fig. 5(b)), to align on the same axis with the LEDs and the photodiode. Thereby, each LED and photodiode was located at the same axis and also the same point on the fingertip during the measurement process (Fig. 5(a)). After that, each LED turned on sequentially and illuminated the tissue for 10s. The system contained a high pass filter that had 0.1Hz cut-on frequency, which was used in order to eliminate offset voltages. The next step was using a 50Hz notch filter which was used for filtering out power network and ambiance light noises. Then, the analog signal was amplified to around 100–1000 using an adjustable gain operational amplifier. Finally, a low pass filter that had a 15Hz cut-off frequency, was used to remove high frequency noise.29 The detected analog signals were converted using an analog digital converter (USB6210, Nat. Inst., USA) and recorded to the personal computer using Labview 2016 software. The sampling rate was set at 1kHz. The PPG signals with six different wavelengths were processed and the absorption spectrum was calculated. In the last step, the optical density and blood glucose concentration relations were investigated.
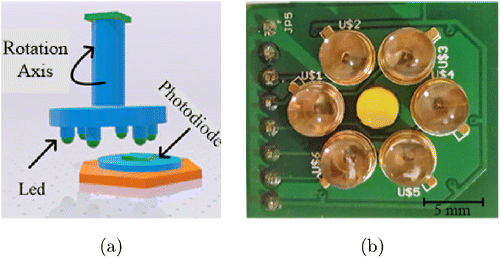
Fig. 5. Positioning of NIR LEDs (a) Schematic view. (b) Actual view of LED placement.
The actual view of the self-designed optical system is shown in Fig. 6. During the measurement process, a fingertip was placed on the photodiode and each LED lit up in turn. The measurement time was set to 10s for each LED.
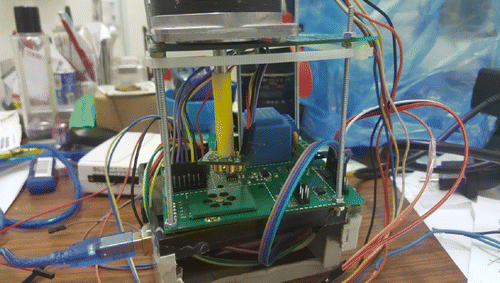
Fig. 6. Actual view of the self-designed optical system.
2.1.2. Ethics statement and volunteers
The ethical protocols of this study were approved by the ethics committee of Ege University Faculty of Medicine (May 25, 2017, No. 17-5/60). The study was conducted in ethical principles. PPG signals of 24 volunteers were recorded by the self-designed optical system. In our experiment, 20 male and 4 female subjects were involved. The average age was 30±9.72 (mean±standard deviation). In addition, their blood glucose levels were measured by a conventional pricking glucometer (GlucoDr, AGM 2200, Allmedicus, South Korea) as a reference. The blood glucose level range of the subjects was between 83–140mg/dl and the average was 104.71±12.58mg/dl. The experiment was done in a darkened room to eliminate ambient light. The volunteers were requested to relax, breath normal, and stay in a resting position. Each subjects measurement took approximately 70–75s.
In the last part of the study, multiple linear regression analysis was applied between the optical absorption values which were acquired through the self-designed system and the reference blood glucose levels. The regression analysis results and the self-designed optical system performance were investigated.
3. Results and Discussion
3.1. Power spectrum density based glucose sensitive optical band determination
The details of the method were given in Sec. 2.1 in which the transmission spectrums were divided into sub-bands and the power spectral density was calculated for each sub-band. Then the power density and the glucose concentration relations were researched. In Fig. 7, the transmission spectrum of the phantom whose optical parameters are μ′s=5.5 and μa=0.1cm−1 is given. Figures 7(a) and 7(b) shows the raw spectrum and its correlations and p values. Figures 7(c) and 7(d) gives pre-processed spectrum and related correlations and p values, respectively.
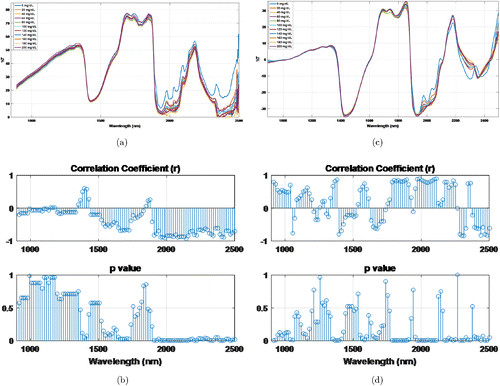
Fig. 7. Different glucose concentrations transmission spectrums of the phantom that has μ′s=5.5 and μa=0.1cm−1: (a) Raw spectrums. (b) Correlations with glucose. (c) Preprocessed spectrums and (d) Correlations with glucose.
The same method was applied for all the phantoms, and averaged absolute correlation coefficients (AACC) against 100nm sub-bands were given in Fig. 8. Here, we propose two representative sub-bands. One of them is the 2100–2200nm band which is also meaningful because it is the combination band of the glucose molecule. The second one is the 1200–1300nm band due to the fact that it has a high correlation coefficient and low standard deviation. For the sake of the cost of the light sources and detectors, the latter sub-band was feasible to study. Also, this study was based on working within this sub-band.
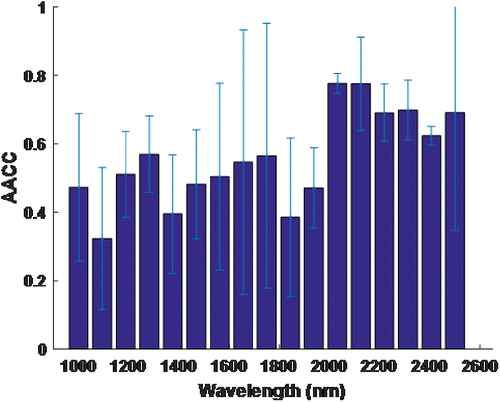
Fig. 8. Absolute averaged correlation coefficient (AACC) against wavelength for the sub-bands.
3.2. PPG based optical system design results
Randomly selected raw PPG signals that were acquired via the self-designed optical system for each 6 different wavelengths from the subject space are shown in Fig. 9.
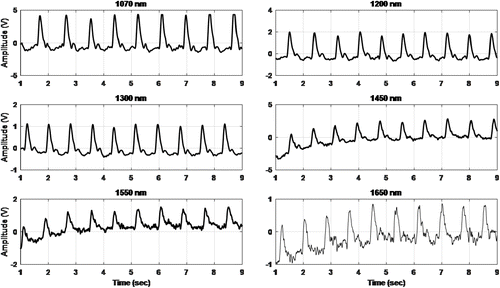
Fig. 9. Raw PPG signals for the 6 different wavelengths.
To enhance the signals, first the Saviztky Golay filter, which is a polynomial moving average filter for smoothing, was applied. After that, a baseline removing filter was applied to eliminate the noise due to breathing. The filtered PPG signals are shown in Fig. 10.
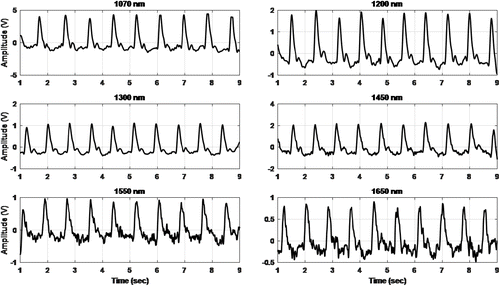
Fig. 10. The filtered PPG signal for six different wavelengths.
To find out the quality of the signals, the signal to noise ratio (SNR) was calculated, and high SNR value signals were used in the prediction model (Fig. 11). Almost all power from the PPG signal was in the 0.5–5Hz range.29 Higher frequencies than this range can be assumed to be noise.
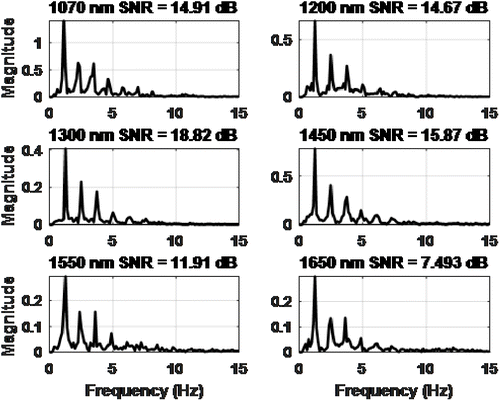
Fig. 11. The spectrum of PPG signals and SNR values.
The performance of the pre-processing is given in Fig. 12. The averaged peaks and the averaged valleys were shown by red dashed lines in the graphs. The raw signal standard deviations which were declared at the top of the graphs, turned into smaller deviations after pre-processing. It was another advantage of the pre-processing.
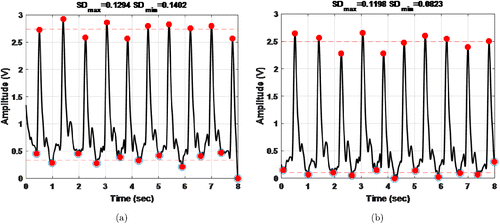
Fig. 12. Detection of PPG signal peak and valley points (a) Raw signal and their peaks (b) pre-processed signal and their peak values SD: standard deviation.
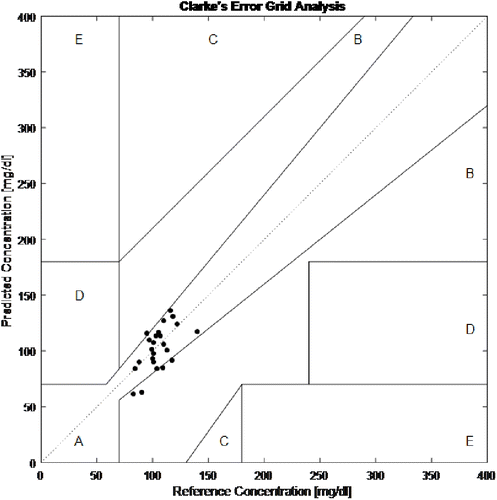
Fig. 13. The spread of prediction values on the Clarke Error Grid Analysis.
Finally, when the optical absorption densities were found for each subject, the multiple linear regression analysis was applied and its results are given below. The optical absorption densities of the 24 subjects for each of the 6 different wavelengths were used as independent variables. The blood glucose level was the dependent variable. The distribution graph of independent and dependent variables was drawn and it was a linear relation so that linear regression analysis was preferred.
A multiple regression analysis with a stepwise method was run to predict blood glucose levels from the 6 different optical density values. All of the independent variables were entered and only 1200 and 1300nm OD values were kept for prediction. This also matched with previous findings from our in-vitro studies. In the regression analysis, there was linearity as assessed by partial regression plots against the predicted values. There was independence of residuals or errors, as assessed by a Durbin-Watson statistic of 2.037. There was not multicollinearity, as assessed by tolerance values greater than 0.1 or variance inflation factors (VIF) smaller than 10. The multiple regression model significantly, and statistically predicted blood glucose levels according to the analysis of variance (ANOVA) test results, where F(2,22)=248.534, p<0.0005, adjusted R2=0.97. While six variables were added, only two of them were statistically significant to the prediction, p<0.0005. Regression coefficients and standard errors can be found in Table 1.
| Prediction method variables (ODλ) | Unstandardized coefficients B (Std. error) | t | p (Significance) |
|---|---|---|---|
| 1200nm | 71.585 (9.341) | 7.664 | 0.000 |
| 1300nm | 49.794 (13.031) | 4.139 | 0.000 |
The equation of the multiple regression model coefficients is given below :
4. Conclusions
In this study, a power spectral density method was proposed for determining representative glucose bands in the NIR spectrum. Different glucose concentrations were added into the prepared tissue phantoms which had different scattering properties. The transmittance spectrums were measured between 900–2500nm range. Each spectrum was divided into 100nm length sub-bands and their power spectral density was calculated. The correlation was investigated between sub-band power density and the glucose concentrations. Two sub-bands were suggested for work on noninvasive blood glucose measurement. One of them was the 2100–2200nm band and the other one was 1200–1300nm band. Furthermore, the 1200–1300nm band was preferred for in-vivo studies because of light source and detector costs and easy procurement. For the in-vivo studies, a low cost, portable, and continuous PPG based optical measurement system was designed. It had six different NIR LEDs placed radially on a rotational axis as a light source, and an InGaAs photodiode as a detector. The fingertips of the subjects were illuminated with six different wavelength LEDs sequentially. The recorded signals were pre-processed, and after that, the averaged peaks and valleys were detected to calculate an optical absorption density. By using a multiple regression analysis with stepwise method, the 1200 and 1300 nm optical density values were determined to statistically significant important variables. The adjusted R2 value was 0.97. The regression analysis results showed that 79.17% of the predictions were in the A zone, and the leftovers were in the B zone according to the Clarke Error Grid Analysis, so that the self-designed optical system can be considered promising and useful tool. In addition, the in-vitro study results and in-vivo regression analysis results were statistically matched. In future studies, an adaptive motion artifact filter can be added and thus can provide robust measurement.
Disclosures
The authors clarify that they have no competing interests.
Acknowledgments
This study was funded by The Scientific and Technological Research Council of Turkey (TUBITAK) under Grant No. 113E610.