Fast repetition rate fs pulsed lasers for advanced PLIM microscopy
Abstract
Simultaneous metabolic and oxygen imaging is promising to follow up therapy response, disease development and to determine prognostic factors. FLIM of metabolic coenzymes is now widely accepted to be the most reliable method to determine cellular bioenergetics. Also, oxygen consumption has to be taken into account to understand treatment responses. The phosphorescence lifetime of oxygen sensors is able to indicate local oxygen changes. For phosphorescence lifetime imaging (PLIM) dyes based on ruthenium (II) coordination complexes are useful, in detail TLD1433 which possesses a variety of different triplet states, enables complex photochemistry and redox reactions. PLIM is usually reached by two photon excitation of the drug with a femtosecond (fs) pulsed Ti:Sapphire laser working at 80MHz repetition rate and (time-correlated single photon counting) (TCSPC) detection electronics. The interesting question was whether it is possible to follow up PLIM using faster repetition rates. Faster repetition rates could be advantageous for the induction of specific photochemical reactions because of similar light doses used normally in standard CW light treatments. For this, a default 2p-FLIM–PLIM system was expanded by adding a second fs pulsed laser (“helixx”) which provides 50fs pulses at a repetition rate of 250MHz, more than 2.3W average power and tunable from 720nm to 920nm. The laser beam was coupled into the AOM instead of the default 80MHz laser. We demonstrated successful applications of the 250MHz laser for PLIM which correlates well with measurements done by excitation with the conventional 80MHz laser source.
1. Introduction
Luminescence lifetime imaging visualizes the decay of a molecule from the first excited singlet state or triplet state. In fluorescence lifetime imaging (FLIM), the lifetime is imaged as the reciprocal of the sum of all rate constants involved in the relaxation process from the first excited singlet state. FLIM of metabolic coenzymes is the basis for optical metabolic imaging (OMI). Intersystem crossing (ISC) from the first excited singlet state to the triplet state is the prerequisite for a spin forbidden process called phosphorescence. The phosphorescence lifetime, which is the reciprocal of the sum of decay rates from the triplet state, is the parameter of interest during phosphorescence lifetime imaging (PLIM). The phosphorescence lifetime can decrease in the presence of quenchers as oxygen. Thus, oxygen which is the most abundant quencher in cells can be determined by PLIM following the Stern–Volmer equation :
OXI by PLIM could be extremely interesting during photodynamic therapy (PDT). Briefly, in PDT, a photosensitizer (PS), used at nontoxic concentrations, undergoes ISC to the triplet excited state after excitation by light and subsequently transfers energy to ground state molecular oxygen (triplet oxygen 3O2). This leads finally to the production of reactive oxygen species (ROS), either singlet oxygen (1O2) by the so-called Type-II reaction or oxygen radicals (Type-I reaction).1 Also, direct oxygen-independent electron transfer reactions to biomolecules are feasible (Type-III reaction).2 As oxygen is critical in PDT, tumor hypoxia is a major problem and could lead to treatment failure. At this point, online monitoring of O2 concentration and O2 consumption could improve PDT treatment protocol. An interesting solution of this demand is to use phosphorescent PSs together with short pulsed laser excitation, which enables both, PLIM imaging and PDT irradiation at pulse intensities with negligible damage to nonstained cells. Increasing the repetition rate of a short pulsed laser compared to the normally used standard 80MHz laser at the same average power and pulse duration decreases two-photon excitation by a factor of √frep180MHz, where frep1 is the repetition rate of a fast repetition rate laser.3 Within this study, investigations where done with a 250MHz repetition rate laser and compared with 80MHz excitation. As will be discussed within this paper, PLIM imaging could be very well achieved at 250MHz excitation rate, however, simultaneous FLIM fitting procedures, recently published with 80MHz excitation rate,4 need further modifications in the detection electronics (see Sec. 2).
As phosphorescent PSs transition metal complexes seem to be promising candidates due to their specific redox behavior and formation of metal-to-ligand charge transfer (MLCT) complexes upon irradiation which opens multiple chemical reaction pathways resulting in cytotoxic redox reactions.5 Besides, they can play a dual role serving as PSs and as phosphorescent dyes.2 Ru(III) complexes, actually in phase II of clinical studies, where ruthenium is in oxidation state III are known to be potential chemotherapeutics in hypoxic areas, due to the reduction of ruthenium (III) to ruthenium (II). This redox activity of ruthenium compounds is believed to represent one major mode of action leading to disturbance of the cellular redox balance in the reductive tumor milieu and induction of apoptosis via the mitochondrial pathway.6
Based on their photophysical and electrochemical properties, Ru(II) complexes have been considered for light-based applications and are under investigation for PDT.7,8,9,10,11,12 The wide range of different triplet excited states makes the Ru(II) complexes attractive. The type of photochemistry depends on the relative energy of the molecular orbitals of the different triplet states, which are reached after ISC from the lowest excited singlet state 1MLCT. The typical MLCT transition is centered around 460nm, other bands in the UV arise from intraligand ππ∗ absorption.7,11 Some compounds in the triplet state undergo photoinduced ligand exchange (from 3LF, Typ III PDT, see Fig. 1), some generate 1O2 (from 3MLCT and 3LC, Typ II PDT, see Fig. 1) or other reactive oxygen species (3LC, Typ I PDT, see Fig. 1).13 Of main interest for the diagnostic purpose is the emissive 3MLCT state, which shows phosphorescence, with a phosphorescence lifetime normally in the sub-μs and μs range, depending on the complex and transition metal used.14,15,16,17,18 For example, the well-known [Ru(bpy)3]2+ (bpy=2,2′-bipyridine) has a lifetime of 620ns in water.13 The compound investigated within this work, TLD1433 (a Ru(II) complex derived from α-terthienyl appended to imidazo [4,5-f][1,10]-phenantroline) has entered a human clinical trial for PDT of nonmuscle invasive bladder cancer (ClinicalTrials.-gov Identifier NCT03053635).7,19 The possible energy levels and transitions in the TLD1433 molecule are presented in the Jablonski diagram in Fig. 1. Recently published results could show that oxygen is the main quencher of TLD1433 leading to a shortening of the phosphorescence lifetime.4 PLIM results reported in this paper were reached by two photon excitations of the drug with a femtosecond (fs) pulsed Ti:Sapphire laser working at 80MHz repetition rate and time-correlated single photon counting (TCSPC) detection electronics. As discussed above, the interesting question now was whether it is possible to follow up oxygen concentration and consumption by PLIM of TLD1433 using higher repetition rates. For this, a 250MHz repetition rate laser was developed and coupled into a standard laser scanning microscope. As mentioned in addition, increasing repetition rates could be advantageous because of decreasing pulse intensities and therefore inducing less damage. The potential impact of fast repetition rate laser excitation for PDT treatment protocols and the development of novel diagnostic approaches towards personalized theranostic procedures will be discussed.
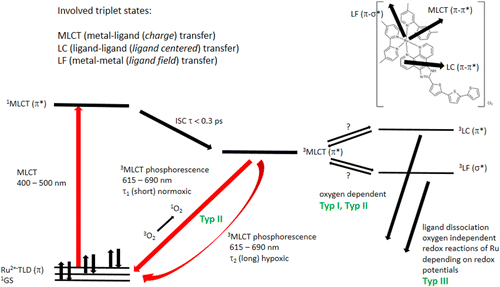
Fig. 1. A possible Jablonski diagram of Ru(II) complexes.
2. Materials and Methods/Experimental
2.1. PLIM measurements
The experimental setup for FLIM–PLIM is based on a multiphoton laser scanning microscope (Zeiss LSM 710 NLO, Carl Zeiss, Jena, Germany) in combination with advanced multidimensional TCSPC technique. We expanded our default configuration by a second fs laser as shown in Fig. 2(a).
In the default FLIM–PLIM configuration, we use the Mai Tai AX HPDS fs laser (Spectra Physics, Darmstadt, Germany) with a repetition rate of 80MHz.20 The laser beam is coupled into an acousto-optical modulator (AOM). The AOM modulates the laser intensity allowing for laser beam ON/OFF modulation, beam blanking and power control. While the latter two are integrated in the Zeiss LSM 710 standard functionality, the external ON/OFF function requires a Zeiss custom modification of the AOM (Zeiss INDIMO Becker & Hickl PLIM). The ON/OFF modulation is essential for recording PLIM in a two–photon laser scanning system. We used fast and sensitive hybrid photodetectors (HPM-100-40, GaAsP cathode, detection range from 300nm to 700nm, Becker & Hickl GmbH, Germany) to detect single photons in a nondescanned detection (NDD) arrangement. NDD allows for best possible collection efficiency in the given setup. Fluorescence and the phosphorescence signals were spectrally separated by a beam-splitter with a 565nm transition wavelength. A 460/60nm band-pass filter and a LP 615 long-pass filter (both AHF Analysentechnik, Tübingen, Germany) were used to clean up the signals.
Concurrent FLIM–PLIM functionality is mainly provided by the TCSPC system consisting of two parallel SPC-150 TCSPC modules and a digital delay generator DDG-210 (all devices Becker & Hickl GmbH, Germany).21 The DDG triggers the ON/OFF function of AOM synchronously to the pixel clock signal of the LSM 710 NLO in order to avoid aliasing effects. Thus, triggered by the DDG, the beam is switched on for few microseconds and excites fluorescence as well as phosphorescence in the sample. FLIM is measured during the ON period, PLIM mainly during the OFF period. All signals are processed by the TCSPC modules and SPCM operating software provided by the manufacturer. This results in a concurrently recorded FLIM–PLIM dataset in two spectral detection channels.
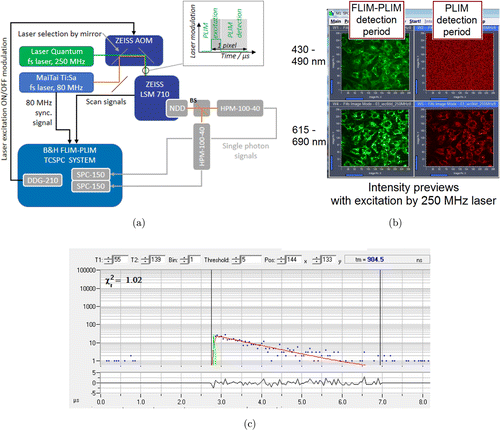
Fig. 2. Schematic of the two-photon FLIM–PLIM setup employing either an 80MHz or 250MHzfs laser, Zeiss LSM 710 with AOM, fast single photon counting HPM-100-40 detectors and FLIM–PLIM TCSPC system. In this mode, the 80 MHz laser only provides the synchronization signal. (b) Intensity preview images during measurement with the 250MHz laser. Left and right: photons collected during the PLIM excitation and detection period, respectively. Spectral detection was done in the range from 430nm to 490nm (upper row) and 615nm to 690nm (lower row). Images show TLD1433-stained monolayered IEC-6 cells. (c) A phosphorescence decay curve in a single pixel of a PLIM-image of the 615–690nm spectral channel.
We expanded our default 2p-FLIM–PLIM system by adding a second fs pulsed laser (“helixx”, Laser Quantum, Konstanz, Germany). This laser provides 50fs pulses at a repetition rate of 250MHz, more than 2.3W average power and is tunable from 720nm to 920nm. For all experiments presented in this paper, the center wavelength of the laser was tuned to 810nm. A prechirper module (Laser Quantum) was added to the laser to compensate for the microscope’s group velocity dispersion and ensure short pulses at the sample location. As shown in Fig. 2(a), the laser beam was coupled into the AOM instead of the default 80MHzfs laser.
We adjusted the AOM transmission to a few percent. This resulted in the application of 6.7mW average power in the sample plane at 810nm with the 250MHz laser. This power level is higher compared to those used with the 80MHz laser. The reason is that the efficiency of two-photon excitation decreases with increasing repetition rate at the same average power and pulse duration. Thus, to achieve the same excitation efficiency, the average power must be higher by a factor of √25080=1.8 (see Sec. 1).
In order to maintain maximum compatibility with our default setup based on the 80MHz fs laser (Fig. 2(a)), we used its 80MHz synchronization signal for the TCSPC electronics. When the 80 MHz laser is used, this arrangement provides the full concurrent FLIM–PLIM functionality. In opposite, with the 250MHz laser, the TCSPC acquisition is not synchronized to the actual fs laser pulses during the excitation period. Thus, no TCSPC-FLIM was recorded yet and only PLIM data are reported in this work. However, in principle, it is possible to record FLIM also with a repetition rate of 250MHz and this would improve the application of this laser. Therefore, we are currently in a dialogue with manufacturers of the hardware considering the best technical solution for simultaneous FLIM–PLIM using fast repetition rate lasers.
We selected an image resolution of 256×256 pixels with 256 time channels in each pixel for primary data collection. We reduced the scan speed to obtain a pixel dwell time of 12.61μs (7.75s per frame) in order to record the relatively slow phosphorescence decay. The laser on time at the beginning of each pixel was 2.8μs, the time range for the PLIM recording was 12.8μs. The total acquisition time was 22.5s, corresponding to the accumulation of 3 frames. The objective lens was an EC Plan-Neofluar 40×1.30 oil (Carl Zeiss, Germany), providing a scan area of 212.1μm×212.1μm at zoom=1.
2.2. Cell culture studies
As a cell model, the crypt-like small intestinal cell line IEC-6 (ATCC® CRL-1592TM), normal rat intestinal epithelial cells, which has been shown to be useful for studies under low O2 as well as normal room oxygen conditions,22,23 was used. The cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, cat.-no. c4359.0500, Genaxxon bioscience GmbH, Ulm) supplemented with GlutaMAXTM Supplement (Thermo Fisher Scientific, Waltham, MA USA), 10% fetal bovine serum (FBS) (Biochrom GmbH, Berlin, Germany) and 0.1 Unit/mL bovine insulin at 37∘C and 5% CO2. For microscopy, all cells were seeded on glass bottom microwell dishes with a coverglass (Greiner Bio-One GmbH, Frickenhausen, Germany) at a density of 150 cells/mm2 and were allowed to grow for 48h in the incubator.
Microscopic observations were performed in medium with all supplements. Before the measurements, the medium in the glass bottom dishes was changed to remove remaining unbound TLD1433 from samples incubated with it. After removing the incubation medium and rinsing twice with medium containing all supplements, the samples stayed at least 2h under standard conditions for cell cultivation (37∘C, 5.0% CO2). For imaging, the same temperature and gas compositions were used. For establishing hypoxic conditions, an oxygen control device was used (O2 Module, Carl Zeiss, Jena, Germany). Hypoxia was induced by decreasing the O2 level from 19.5% of oxygen to 0.0%, substituting oxygen with nitrogen during the 2h incubation. The level of CO2 stayed at 5.0% for both oxygen conditions.
TLD1433 was a gift from Theralase Technologies Inc. (Toronto, Canada). The structure and synthesis were published in Ref. 24. TLD1433 is a Ru(II)-based complex with a general structure of [Ru(LL)2(LL′)]2+, where LL is 4,4′-dimethyl-2,2′-bipyridine (dmb) and LL′ is 2-(2′,2′′:5′′,2′′′-terthiophene)-imidazo[4,5-f][1,10] phenanthroline (IP-TT). TLD1433 diluted in water was applied to cell monolayers in medium for cell cultivation in a final concentration of 20μM 24h before PLIM measurements.
2.3. PLIM data and statistical analysis
We measured the phosphorescence emitted by TLD1433 within the living IEC-6 cells. The spectral detection range of phosphorescence was 615nm to 690nm when excited with 810nm.
To obtain phosphorescence lifetimes, we fitted phosphorescence decay curves using the SPCImage software (Becker & Hickl GmbH, Berlin, Germany) with a monoexponential decay model given by
For statistical evaluation, three different samples with monolayered cells in the dishes were performed applying the “helixx” laser as well as the of MaiTai laser. In total, 18 single images from 3 different samples, where every image contained about 30–40 cells, were measured for every laser source and oxygen condition.
Statistical evaluation was performed using GraphPad Prism 7 for Windows (GraphPad Software, Inc., La Jolla, USA). For all PLIM data, a normal distribution was verified by D’Agostino & Pearson omnibus normality test. Unpaired two-tailed t-test was used for comparison procedure. Statistically significant normal distributions, equal variances and equalities of two means were predefined with a significance level of α=0.001.
3. Results and Discussion
The PLIM setup presented in the Fig. 2(a) was designed to detect the phosphorescence of TLD1433 within the spectral channel 615nm to 690nm. The emission spectrum of TLD1433 under two-photon excitation was measured in solution as well as within living cells and presented previously.4,25 Similar to other ruthenium (II) complexes investigated by us, the tris-(2,2′-bipyridyl) dichloride (Ru(bpy)2+3) and cHSA-PEO-TPP-Ru, TLD1433 emits in the range from 550nm and 670nm.20,26
We were able to perform two-photon microscopy using the 250MHz Ti:Sa on a Zeiss LSM 710 NLO. We found that excitation at 810nm is suitable for TLD1433 and other ruthenium complexes.26 An advanced TCSPC system controlled a specific concurrent FLIM–PLIM procedure as described previously.4 The TLD1433 phosphorescence with relatively long lifetimes was excited during the “laser on” period and detected during the “laser off” period.
The FLIM and PLIM imaging takes place during “laser on” and “laser off” period, respectively. This provides simultaneous FLIM and PLIM imagining in all spectral channels. Indeed, both spectral FLIM channels in Fig. 2(b) show cellular shape images, which could be explained by autofluorescence of the living cells together with fluorescence of the ruthenium complex TLD1433. Our previously published results of FLIM/PLIM imaging were performed using a 80MHz laser, which generates laser pulses every 12.5ns, enabling simultaneous NADH FLIM and TLD1433 PLIM. The “helixx” laser with 250MHz repetition rate provides a laser pulse every 4ns, which limits the application of the laser to relatively short fluorescence lifetimes up to 3 ns at maximum. This lifetime range still includes many fluorophores such as native intracellular fluorescent coenzymes NADH and FAD+ or dyes like Oregon Green.27 However, due to the high repetition rate of this laser, we are yet not able to record FLIM with our existing electronics. In the future, as a promising solution, TCSPC acquisition will be synchronized to the fs laser pulses during the excitation period of the 250MHz rate laser.
Demonstrating an extremely low dark cytotoxicity, TLD1433, nonetheless, showed a strong photodynamic effect,4,11,28 which imposes restrictions on the luminescence imaging, mostly to the used laser power. The 250MHz laser with a 3.125-fold higher pulse frequency than the 80MHz laser requires a higher laser average power to compensate for the quadratic dependency of the two-photon absorption.3 Within this work, we found that we could increase the applied laser power without enhanced cell destruction from 2mW–3.5mW in the focal plane for the 80MHz laser to about 6.7mW for the 250MHz laser (data not shown).
Figure 3 shows PLIM images of IEC-6 monolayer cells, incubated 24h with 20μM TLD1433 in the presence of serum. The PLIM signal within the channel is entirely attributable to the phosphorescence of TLD1433 with relative long lifetime; the control cells without the dye do not show any emission within this spectral range (data are not shown). A granular distribution of TLD1433 within the cells was demonstrated recently4 and shown to be mainly lysosomal. The phosphorescence lifetime was obtained by a monoexponential fitting procedure and presented in false colors within the range 600–2200ns (Fig. 3, right)
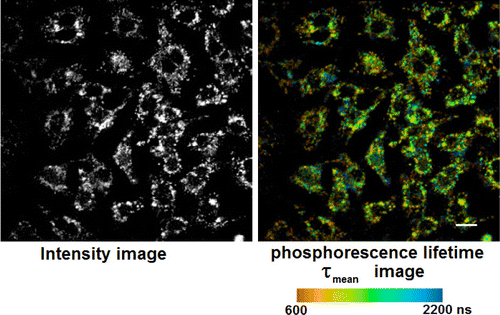
Fig. 3. PLIM of IEC-6 monolayer cells of the 615–690nm spectral channel after excitation with fs pulsed laser at 250MHz repetition rater: Intensity image (left) and in false colors, the phosphorescence mean lifetime in the range from 600ns to 2200ns (right); the scale bar corresponds to 20μm.
The sensitivity of TLD1433 phosphorescence lifetime to the oxygen level was previously published in Ref. 4. Oxygen quenches the phosphorescence, which leads to shortening of the lifetime under enhanced oxygen levels. The statistical evaluation in Fig. 4 shows the mean phosphorescence lifetime of TLD1433 within the cells under different oxygen conditions for two different excitation modes: 250MHz versus 80MHz repetition rate. The results were similar and a significant increase of the value at hypoxia (0.0% oxygen) of about 400ns is verified for both excitations. In conclusion, the compact 250MHz laser leads to comparable results and is of potential value for multiphoton, not only PLIM microscopy.
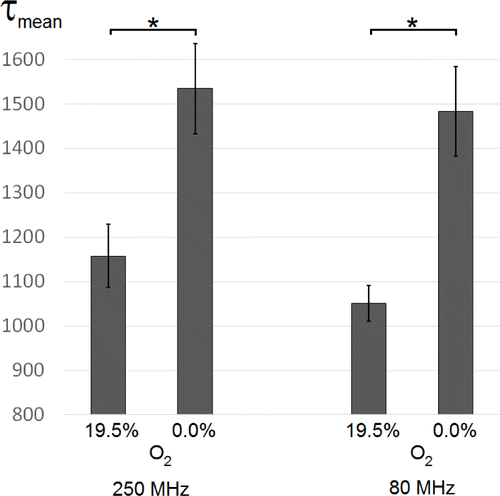
Fig. 4. Statistical evaluation of the phosphorescence lifetime τmean of IEC-6 monolayer cells in nanoseconds previously incubated with TLD1433 under 19.5% oxygen and under hypoxic (0.0% oxygen) conditions, measured by using two different laser modes: 250MHz (left) and 80MHz (right) repetition rate lasers (∗p<0.001, n=18).
4. Conclusion
We have successfully demonstrated the application of a compact 250MHzfs titanium sapphire laser for less invasive two-photon excited microscopy and its potential use for PLIM imaging. Providing future modifications of the TCSPC electronics for fast repetition rate lasers, we see potential applications for simultaneous FLIM/PLIM and monitoring of important metabolic coenzymes, including NADH and FAD+, and oxygen-dependent imaging of phosphorescent probes. Importantly, PLIM imaging of cells incubated with Ru(II) complexes is comparable by using either a standard 80MHz laser or the newly developed 250MHz laser and the lifetime results correlate well. The advantage of the 250MHz laser is the small footprint and the decreased pulse intensity and therefore the less invasive character of the system. The presented setup can be easily expanded by additional spectral channels depending on the requirements.
Conflict of Interest
The authors declare no competing financial interest. Alexander Jelzow is an employee of Becker & Hickl GmbH; Tobias Plötzing is an employee of Laser Quantum GmbH.
Acknowledgments
This work is currently supported by the Ministry of Research and Development, FKZ order: 13N14508 (“OMOXI”) and by the Ministry of Economics, ZIM-Project, FKZ: ZF4322901RE6 (“UFEMPU”). The authors would like to thank Company Theralase Inc. 1945 Queen St. Toronto, ON M4L 1H7 for providing us TLD1433, Kirsten Reeß from the core facility for sustained help with cell culture and the Ulm University Center for Translational Imaging MoMAN for its administrative support.