Aggregation-induced emission luminogen for in vivo three-photon fluorescence lifetime microscopic imaging
Abstract
Compared with visible light, near-infrared (NIR) light has deeper penetration in biological tissues. Three-photon fluorescence microscopy (3PFM) can effectively utilize the NIR excitation to obtain high-contrast images in the deep tissue. However, the weak three-photon fluorescence signals may be not well presented in the traditional fluorescence intensity imaging mode. Fluorescence lifetime of certain probes is insensitive to the intensity of the excitation laser. Moreover, fluorescence lifetime imaging microscopy (FLIM) can detect weak signals by utilizing time-correlated single photon counting (TCSPC) technique. Thus, it would be an improved strategy to combine the 3PFM imaging with the FLIM together. Herein, DCDPP-2TPA, a novel aggregation-induced emission luminogen (AIEgen), was adopted as the fluorescent probes. The three-photon absorption cross-section of the AIEgen, which has a deep-red fluorescence emission, was proved to be large. DCDPP-2TPA nanoparticles were synthesized, and the three-photon fluorescence lifetime of which was measured in water. Moreover, in vivo three-photon fluorescence lifetime microscopic imaging of a craniotomy mouse was conducted via a home-made optical system. High contrast cerebrovascular images of different vertical depths were obtained and the maximum depth was about 600 m. Even reaching the depth of 600 m, tiny capillary vessels as small as 1.9 m could still be distinguished. The three-photon fluorescence lifetimes of the capillaries in some representative images were in accord with that of DCDPP-2TPA nanoparticles in water. A vivid 3D reconstruction was further organized to present a wealth of lifetime information. In the future, the combination strategy of 3PFM and FLIM could be further applied in the brain functional imaging.
1. Introduction
Brain imaging has widely arisen people’s attention since the last century. In most of the imaging techniques, optical imaging is one of the most favourable methods for its high resolution, no invasion or radiation and flexible combination with other imaging techniques.1 However, the excitation wavelength of the common fluorescence imaging usually locates in the visible spectral region, which can only reach the superficial surface of the biological tissues. Since the brain tissue is a high scattering medium,2 near-infrared (NIR) light can get much larger penetration depth than visible light, because of its better anti-scattering ability in biological tissues.3,4 Multi-photon fluorescence microscopic imaging provides a powerful bioimaging approach, which utilizes the NIR light as excitation. In order to achieve a deep penetration, traditional two-photon fluorescence microscopy (2PFM) adopts femtosecond (fs) lasers in NIR-I region (700–900nm).5,6 As fs lasers have developed a lot recently, it is becoming more and more convenient to obtain NIR lasers with longer wavelength and higher power density. Meanwhile, three-photon fluorescence microscopy (3PFM) with 900–1700nm (NIR-II region) fs excitation has been quickly developed.7,8,9 The 3PFM imaging can penetrate deeper in the brain tissue than 2PFM because excitation lasers in the NIR-II region have less photon scattering.10 Besides, the three-photon fluorescence (3PF) with higher order nonlinearity brings up higher spatial resolution, larger signal to background ratio, and better optical sectioning ability.11 Thus, the 3PFM imaging can obtain high-contrast image in the deep tissue.
Although 3PFM has superior imaging performance, the vast majority of endogenous fluorophores have small three-photon absorption (3PA) cross-sections, which cannot be excited effectively. Thus, various types of fluorescent probes are adopted to improve the contrast in the 3PFM imaging.12 However, the metal contained nanoparticles, such as quantum dots, upconversion nanoparticles and gold nanorods, are limited by the excretion difficulty and potential toxicity. The common organic dyes are endowed with high biological compatibility, but limited by small 3PA cross-sections and aggregation-caused quenching property.13 Hence, aggregation-induced emission luminogens (AIEgens) can be considered as alternatives for in vivo 3PFM imaging. As a type of organic dyes, AIEgens have a large 3PA cross-section and enhanced fluorescence when encapsulated in nanoparticles.14 When performing the deep-tissue 3PFM imaging, there are still some limitations, such as the small amounts of probes in the targeted samples and the weakened fluorescence intensity at a large depth. Thus, the images of these areas are lack of enough contrast. This could be improved by combining the 3PFM imaging with the fluorescence lifetime imaging microscopy (FLIM), which is suitable for noninvasive study of intracellular processes,15,16 microfluidic systems,17 remote sensing,18,19 lipid order problems in physical chemistry,20 temperature sensing21 and clinical medicine.22 FLIM can provide a more sensitive and precise image based on the weak signals, compared with the traditional fluorescence intensity imaging.23 One reason is that the fluorescence lifetime of probe keeps stable at different amounts or under varying intensity of the excitation laser.24,25 Another reason is that the fluorescence lifetime of each pixel is obtained with time-correlated single photon counting (TCSPC) technique, thus with a significantly increasing signal–noise ratio.26
In this paper, DCDPP-2TPA, a novel AIEgen, was adopted as fluorescent probe. The three-photon absorption cross-section of the AIEgen, which has a deep-red fluorescence emission, was proved to be large. The absorption spectrum and the 3PF spectrum of AIEgen nanoparticles were both measured and recorded. The 3PF lifetimes of AIEgen nanoparticles in water and AIEgen molecular in different solvents (THF, chloroform and toluene) were measured. Besides, a 3PF lifetime imaging system based on the TCSPC technique was set up to conduct the further imaging. By injection with AIEgen nanoparticles, brain vasculature of one skull-removed mouse was imaged and the maximum depth reached about 600m. Even reaching the depth of 600m, tiny capillary vessels (m) could be distinguished. In addition, the 3PF lifetimes of AIEgen nanoparticles at some representative depths were in accord with the previous results. Finally, a vivid 3D reconstruction was further obtained and presented a wealth of lifetime information. In the future, the combination strategy of 3PFM imaging and FLIM could be further applied in the brain functional imaging and provide a larger detection depth.
2. Experimental Section
2.1. Materials
The AIEgen molecule, 5,6-bis(4’-(diphenylamino)-[1,1’-biphenyl]-4-yl)pyrazine-2,3-dicarbonitrile (DCDPP-2TPA), was prepared via our previous study.8 Ethanol, chloroform, toluene and tetrahydrofuran (THF) were purchased from Sinopharm Chemical Reagent Co., Ltd. (SCRC). Pluronic F-127 was purchased from Sigma-Aldrich. Other chemical reagents without specific mention were also purchased from SCRC. Throughout the experiments, deionized (DI) water was commonly used.
2.2. Preparation of DCDPP-2TPA nanoparticles
The AIE nanoparticles were synthesized in the following way. An aliquot of 5mL chloroform solution containing 2.2mg DCDPP-2TPA and 26.4mg F-127 was added into a flask first and the flask was put into the ultrasonic cleaner for 5min to ensure even mixing. The flask was then placed in a rotary evaporator to evaporate all liquid. Until the chloroform was completely removed, 2.2mL DI water was used to dissolve the nanoparticles in the flask. After that, the flask containing aqueous dispersion of AIEgen nanoparticles was put into the ultrasonic cleaner again and sonicated for another 5min. At last, a clear aqueous dispersion of DCDPP-2TPA nanoparticles was gathered.
2.3. Characterizations of DCDPP-2TPA nanoparticles
The size distribution of AIE nanoparticles synthesized by us was measured via dynamic light scattering (DLS) using a particle size analyzer (90 Plus, Brookhaven Instruments Co., USA). The zeta potential of nanoparticles was recorded with the same instrument. The morphology of nanoparticles was observed via a transmission electron microscope (TEM, JEM-1200EX, JEOL, Japan). Both extinction and transmission spectra (300-1800nm) were measured using an UV–Vis–NIR scanning spectrophotometer (Agilent Cary 5000).
2.4. Cell viability analysis
The cytotoxicity of DCDPP-2TPA nanoparticles was evaluated via the instruction of cell counting kit-8 (CCK-8, Beyotime). About 20,000 cells/well in a 100L suspension were incubated in 96-well plates for 24h. A 100L HBSS (Gibco) containing nanoparticles of different concentrations was then added into each well. After incubated for 1h, the culture medium was removed and the cell well was washed three times with HBSS. An aliquot of 100L culture medium containing CCK-8 (10%) was added into each well and incubated for another 4h. Finally, the absorbance at 450nm and 625nm was measured with a microplate reader (Thermo, USA).
2.5. Animal preparation
All of the animal experiments and housing procedures were conducted strictly according to the requirements of the Institutional Ethical Committee of Animal Experimentation of Zhejiang University.
2.6. The one-photon and three-photon fluorescence measuring system
A home-made optical system was used to measure 1PF and 3PF spectra of AIEgen nanoparticles. 1PF was excited by a continuous wave (CW) laser with a center wavelength of 450nm and 3PF was excited by a fs laser (FLCPA-01C, Calmar Laser, 400fs, 1MHz) with a center wavelength of 1550nm. The aqueous dispersion of AIEgen nanoparticles was put into a cuvette. The cuvette was placed near the focus of a lens (mm) to receive the focussed laser. For limiting self-absorption, the focus of the laser beam was further adjusted to be close to the inner surface of cuvette. Then the 1PF or 3PF signals were collected at a 90∘ angle by an objective (XLPLN25XWMP2, Olympus, , working mm, ). Finally, the fluorescence signals were recorded by an optical fiber spectrometer (PG 2000, Ideaoptics Instruments). More details of this optical system can be found in our previous work.27
2.7. The three-photon fluorescence lifetime microscopic imaging system
The fs laser beam with a center wavelength of 1550nm was divided into two perpendicular directions by a polarization beam splitter (PBS), adopted to excite the 3PF and trigger the time synchronization of TCSPC system, respectively (Scheme 1). A quarter waveplate was put before the PBS in order to adjust the power of laser beam in the two divided directions. The stronger laser beam was introduced into a scanning microscope (FV1200&BX61, Olympus) and reflected by an 800nm short-pass dichroic mirror (DC) to excite AIEgen nanoparticles. The focus was adjusted by controlling the -axis of the water-immersed objective (XLPLN25XWMP2, Olympus, , working mm, ) on computer. The 3PF signals collected by the water-immersed objective passed through the DC and were captured by a photomultiplier tube (PMT, HPM-100-50, Becker&Hickl GmbH).
The weaker laser beam was utilized as synchronization signals via a photodetector (PD) in the TCSPC system (Becker&Hickl GmbH, SPC-150). The 3PF lifetime imaging system was used to measure fluorescence lifetimes of AIEgen nanoparticles in water and AIEgen molecular in different solvents (THF, chloroform and toluene). Furthermore, the system was adopted to conduct the three-photon fluorescence lifetime imaging microscopy (3PFLIM) on mouse. A healthy ICR mouse underwent craniotomy surgery and the open window of the skull was protected by a clean glass cover. Before imaging, the mouse under anesthetic was intravenously injected with 200L AIEgen nanoparticles in PBS (1mg/mL). During the imaging, the mouse was immobilized on the stage.
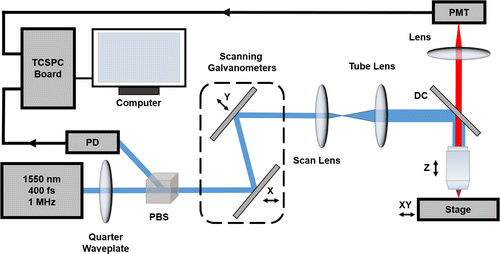
Scheme 1. Schematic illustration of 3PF lifetime microscopic imaging system.
3. Results and Discussion
3.1. Preparation of DCDPP-2TPA nanoparticles
The chemical structure of DCDPP-2TPA was shown in Fig. 1(a). DCDPP-2TPA was encapsulated into F-127, one kind of amphiphilic polymer. DCDPP-2TPA nanoparticles were obtained, as shown in Fig. 1(b). The size distribution and TEM image of these AIEgen nanoparticles (Fig. 1(c)) indicated an average size of approximately 40nm, which means that these nanoparticles were suitable for circulation in blood vessels in the further bioimaging.28 The zeta potential of DCDPP-2TPA nanoparticles was about –mV. In addition, DCDPP-2TPA nanoparticles showed negligible toxicity toward cells (Fig. 1(d)).
3.2. Optical characterization of DCDPP-2TPA nanoparticles
As shown in Fig. 1(e), the extinction spectrum and the transmission spectrum (the inset) of AIEgen nanoparticles in water (0.03mg/mL) were recorded. The spectra indicated that the AIE nanoparticles at 1550nm have very low one-photon absorption. According to our previous study, the 3PA cross-section of DCDPP-2TPA molecule was cm6 s2 at 1550nm, much larger than most commonly used three-photon fluorescent probes (Rh6G, 0cm6 s2 (at 1300nm); Fluorescein, cm6s2 (at 1300nm); wtGFP, cm6 s2 (at 1300nm)).8,29,30,31,32 Besides, TPATCN molecule has no nonaromatic double bonds, and nanoparticles composed of substantial TPATCN molecules showed higher stability than most existing AIE NPs under high-power laser excitation.8,33 One-photon and three-photon fluorescence spectra of DCDPP-2TPA nanoparticles in water (1mg/mL) excited with a CW laser centered at 450nm and a fs laser centered at 1550nm, respectively, were recorded (Fig. 1(f)). The fluorescence spectra with one-photon and three-photon excited were almost the same. The central wavelength of the fluorescence spectra was 645nm.
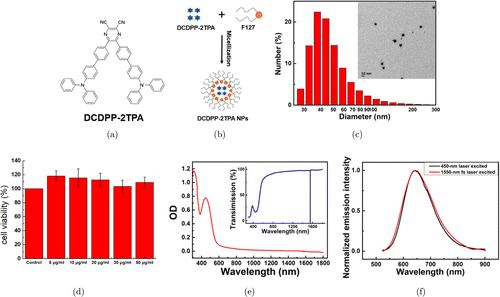
Fig. 1. (a) Chemical structure of DCDPP-2TPA. (b) Preparation scheme of DCDPP-2TPA nanoparticles. (c) DLS result of DCDPP-2TPA nanoparticles in aqueous dispersion (inset: a typical TEM image of DCDPP-2TPA NPs. Scale bar: 50nm). (d) Viability of HEK293t cells after incubation with DCDPP-2TPA nanoparticles of various concentrations for 24h. (e) Extinction spectrum of DCDPP-2TPA nanoparticles in water (1mg/mL). OD, optical density (inset: the transmission spectrum of DCDPP-2TPA nanoparticles in water (1mg/mL)). (f) One-photon and three-photon fluorescence spectra of DCDPP-2TPA nanoparticles in water (1mg/mL).
3.3. Measurement of three-photon fluorescence lifetime
DCDPP-2TPA was directly dissolved in tetrahydrofuran, chloroform and toluene without encapsulation. The 3PF lifetimes of DCDPP-2TPA in these three solutions were measured, which were 570 ps in tetrahydrofuran, 960ps in chloroform and 3366ps in toluene (Fig. 2(a)). Compared with them, 3PF lifetime of DCDPP-2TPA nanoparticles in water was around 5215ps, relatively longer since the aggregated state of DCDPP-2TPA molecules in nanoparticles was rather compact.34 Three-photon fluorescence intensity image (in grayscale) and three-photon fluorescence lifetime image (quadrate color map) of DCDPP-2TPA nanoparticles in water (contained in a glass capillary tube) were both recorded (Fig. 2(b)). The distribution of 3PF lifetime within the color map region (in Fig. 2(b)) was shown in Fig. 2(c), and the central lifetime was measured to be 5.2ns.
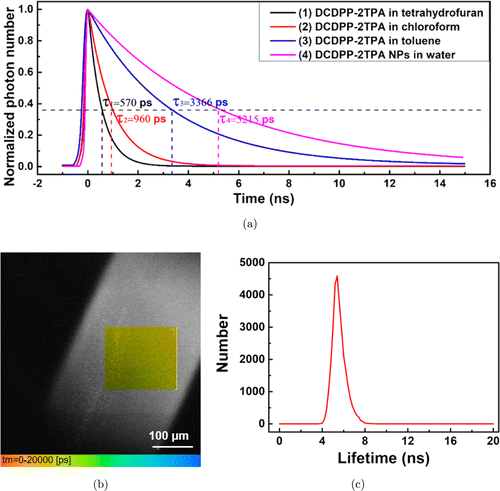
Fig. 2. (a) Time-resolved decay profiles of 3PF of DCDPP-2TPA in tetrahydrofuran (black line), chloroform (red line), toluene (blue line), and DCDPP-2TPA nanoparticles in water (pink line). The lifetimes of each solution were measured to be 570, 960, 3366 and 5215ps. (b) Three-photon fluorescence intensity image (in grayscale) and three-photon fluorescence lifetime image (quadrate color map) of DCDPP-2TPA nanoparticles in water contained in a glass capillary tube. (Scale bar: 100m) (c) Distribution of lifetime within the region of color map in (b).
3.4. In vivo three-photon fluorescence lifetime microscopic imaging
In vivo 3PFLIM was further conducted, and DCDPP-2TPA nanoparticles were used as the three-photon fluorescent probes to stain the brain vasculature of mice. An eight-week-old male mouse with craniotomy was anesthetized first and then intravenously injected with 200L aqueous dispersion of DCDPP-2TPA nanoparticles (1mg/mL, PBS, ). The mouse was imaged by the aforementioned 3PF lifetime microscopic system under fs laser (with a center wavelength of 1550nm) excitation, and the fluorescence lifetime images of brain vasculature at different depths were obtained. Cerebrovascular 3PFLIM images were recorded at every 20m and the penetration depth of blood vessels reached about 600m. 3D reconstructions of brain vasculature were further built with the software of Imaris (Fig. 3).
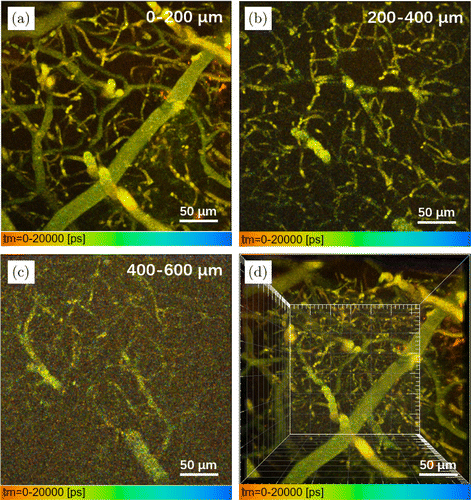
Fig. 3. In vivo 3PFLIM stacked images (every 200m) of mouse with craniotomy brain vasculature at various vertical depths: (a) 0200m, (b) 200400m and (c) 400600m. (d) 3D reconstruction of the blood vessels in brain with 600m depth. (Scale bar: 50m).
3PFLIM images of brain vasculature at different depths were recorded, and some specific images (0, 200, 400, 600m) were shown in Figs. 4(a), 4(d), 4(g) and 4(j). In order to confirm whether the fluorescence signals came from the DCDPP-2TPA nanoparticles and whether the fluorescence signals in vivo have changed, the 3PF lifetimes of certain spots of the blood vessels in these images were analyzed, which were 4086ps at 0m, 4656ps at 200m, 4646ps at 400m and 6470ps at 600m (Figs. 4(b), 4(e), 4(h) and 4(k)). Although DCDPP-2TPA nanoparticles distributed at various depths in the mouse brain, their fluorescence lifetimes were still stable, ranging from 4ns to 7ns, which were within the 3PF lifetime distribution of DCDPP-2TPA nanoparticles in vitro (Fig. 2(c)). This fact illustrated that DCDPP-2TPA nanoparticles could be a stable probe to be utilized in in vivo 3PFLIM and keep the same aggregated state both in vivo and in vitro. Afterwards, to evaluate the spatial resolution of 3PF lifetime images, a line was drawn across capillary vessels in each image at different depths, and the full width at half-maximum (FWHM) of the intensity curve was calculated by Gaussian fitting (Figs. 4(c), 4(f), 4(i) and 4(l)). At the depth of 600 m, tiny capillary vessels (m) could still be distinguished clearly.
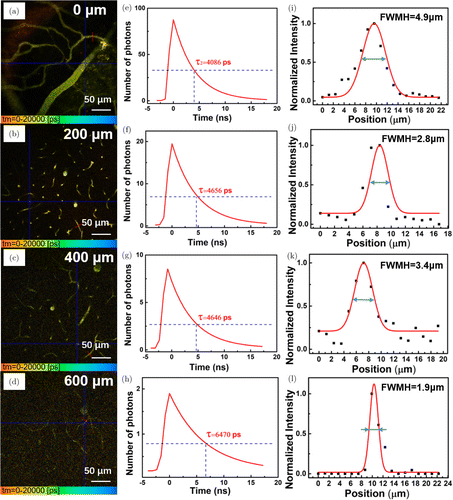
Fig. 4. ((a), (d), (g), (j)) In vivo 3PFLIM images of brain vasculature of mouse with craniotomy at various vertical depths: 0, 200, 400 and 600m. (Scale bar: 50m) ((b), (e), (h), (k)) Time-resolved decay profiles of the intersection of blue lines (drawn in ((a), (d), (g), (j))) at various vertical depths. ((c), (f), (i), (l)) The cross-sectional intensity profiles across the capillary vessels marked with red-dashed lines in ((a), (d), (g), (j)).
4. Conclusions
In summary, aqueous dispersion of AIEgen (DCDPP-2TPA) nanoparticles was synthesized and characterized. Moreover, three-photon fluorescence lifetime imaging microscopy (FLIM) was realized by combining time-correlated single photon counting (TCSPC) technique and three-photon fluorescence microscopic system. Three-photon fluorescence lifetimes of AIEgen in different solvents and AIEgen nanoparticles in water were obtained basing on this setup. Afterwards, with the fs laser (with a center wavelength of 1550nm) excitation and AIEgen nanoparticles, the vivid 3D reconstruction of three-photon FLIM cerebrovascular imaging of a skull-opened mouse was obtained by the 3PFLIM, and the imaging depth reached 600m. 3PF lifetimes of nanoparticles in the living mouse were the same as that measured in vitro, showing that DCDPP-2TPA nanoparticles had the same aggregated state both in vivo and in vitro. Furthermore, tiny capillary vessels as small as 1.9m could be distinguished clearly on the bottom of the image (600m). Three-photon FLIM can achieve in vivo 4D (3D + lifetime) brain structural and functional imaging, which increases lifetime information while ensuring high resolution and deep penetration.
Conflict of Interest
The authors declared that there was no conflicts of interest in this work.
Acknowledgment
This work was supported by National Natural Science Foundation of China (61735016) and Zhejiang Provincial Natural Science Foundation of China (LR17F050001).