Detecting benign uterine tumors by autofluorescence lifetime imaging microscopy through adjacent healthy cervical tissues
Abstract
The endogenous fluorophores such as reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) and flavin adenine dinucleotide (FAD) in cells and tissues can be imaged by fluorescence lifetime imaging microscopy (FLIM) to show the tissue morphology features, as well as the biomolecular changes in microenvironment. The two important coenzymes in cellular metabolism, NAD(P)H and FAD, can be used to monitor the cellular metabolic status. This work proposed a novel method to study the uterine metabolism at the adjacent site of healthy cervix. It was found that the benign uterine tumors such as leiomyomas and adenomyosis with abnormal cell growth can be detected by measuring the fluorescence lifetime of NAD(P)H and FAD in adjacent healthy cervical tissues. This method opened a novel strategy for afflicted women to undergo the cervical biopsies instead of hysterectomies for detecting tumors, which can preserve the fertility of patients. The FLIM studying on NAD(P)H and FAD indicated the correlation between metabolism and some diseases, including diabetes, hyperthyroidism and obesity. It was also suggested that the metabolic level might be quite different for a patient with a malignant tumor history.
1. Introduction
Optical imaging techniques especially fluorescence lifetime imaging microscopy (FLIM) are promising for the detection of malignancies1,2,3,4,5,6 Differentiating malignancies from normal tissues by autofluorescence can rely on the biochemical and tissue morphological changes. The traditional gold standard technique for tissue characterization is haematoxylin and eosin (H & E) stained histopathology, based on morphological changes. However, biochemical change occurs when the cellular metabolism becomes abnormal in the case of rapid cell division, which occurs before morphological changes become apparent.7,8 That means optical techniques have the great potential to detect malignancies or precancer much earlier than traditional histopathology.
It is well known that the endogenous fluorophores in cells and tissues include reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H), flavin adenine dinucleotide (FAD), collagen, and elastin.9 The structural proteins, mainly collagen and elastin, can show the extracellular matrix of tissues reflecting the tissue morphology and structure.10,11 While NAD(P)H and FAD are known as classic molecules involving in oxidative phosphorylation and glycolysis,12,13,14,15 which can be used to describe the cellular metabolic environment in tissues. Based on FLIM, different cancers including cervical cancer,4 lung cancer,6 oral cancer,16 breast cancer,17 were studied on the autofluorescence for early detection. Nevertheless, little attention was paid on benign tumors so far.
Uterine leiomyomas (fibroids) are common gynecologic benign tumors in women, whose rate were reported more than 50–80% of reproductive-aged women.18 Adenomyosis is another common benign gynecological disease. The estimates of its incidence vary widely and were reported from 5% to 70%,19 with the mean frequency of adenomyosis at hysterectomy given as approximately 20–30%.20 The confirmed diagnosis of these common diseases for women currently requires minimally invasive surgery or hysterectomy to obtain the uterine tissues.21 However, minimally invasive surgery is generally not suitable for patients with adenomyosis. Furthermore, hysterectomy is contraindicated for women with a desire to preserve their fertility. For this reason, new technologies are needed to aid the detection of benign tumors in uterus. If this could be achieved at the very early stage of the benign tumor development, drug therapy can be prescribed and administered continuously as an alternative to surgery.
Since NAD(P)H and FAD are important coenzymes in cellular energy metabolism that can emit fluorescence, the abnormal metabolism can be monitored by FLIM. Though the benign tumor develops in uterus, the cellular metabolic status at the adjacent site of cervix may be affected and revealed by FLIM.
In this study, we proposed a novel method for detecting benign uterine tumors such as leiomyomas and adenomyosis at the adjacent site of healthy cervix through fixed cervical tissue samples. It is very promising to apply FLIM method to identify benign tumors in uterus by means of cervix biopsies. It suggested an approach to avoid surgery for benign tumor detection.
2. Materials and Methods
2.1. Participants and sample preparation
The involved 27 patients were from the Central Hospital of Wuhan, Hubei, China, who underwent hysterectomy or biopsy between May 2017 and March 2019. This work was approved by the Institution Review Board of the Central Hospital of Wuhan, and all the involved patients provided informed consent to participants.
The histologic diagnoses were provided by Department of Pathology, the Central Hospital of Wuhan. The samples comprised 17 patients undergoing hysterectomy, including 8 women with uterine leiomyomas and 9 women with adenomyosis or coexisting with leiomyomas. The control (normal) group included 10 women who underwent biopsies with a histologic diagnosis of healthy uterus and cervix. The average age of all the patients was 50.8±8.6 years old, ranging between 35 and 71 years old. The participants had received at least one health examination before the gynecological surgery or biopsy, including blood pressure (BP), thyroid hormones (THs) and thyroid-stimulating hormone (TSH) tests, blood glucose (Glu), body mass index (BMI). Their personal medical histories were also recorded. Table 1 lists the ages, chronic diseases that affect metabolism, and medical history of patients.
| Age | FLIM system | ||||
|---|---|---|---|---|---|
| Patient No. | (Years old) | Diagnosis | (Single/Two-channel) | Chronic diseases | Surgery history |
| 1 | 35 | Normal | Single-channel | ||
| 2 | 41 | Normal | Single-channel | EP (**14 months later) | |
| 3 | 44 | Normal | Single-channel | ||
| 4 | 52 | Normal | Single-channel | ||
| 5 | 61 | Normal | Both | OWT | BC (2007), CA (2010), BCM (2013) |
| 6 | 41 | Uterine leiomyoma | Both | OWT | |
| 7 | 43 | Uterine leiomyoma | Single-channel | OWT | |
| 8 | 48 | Uterine leiomyoma | Both | ||
| 9 | 48 | Uterine leiomyoma | Both | ||
| 10 | 49 | Uterine leiomyoma | Single-channel | ||
| 11 | 53 | Uterine leiomyoma | Single-channel | HTN | |
| 12 | 61 | Uterine leiomyoma | Single-channel | DM | |
| 13 | 71 | Uterine leiomyoma | Both | HTN, DM, HT | |
| 14 | 41 | Uterine adenomyosis | Single-channel | ||
| 15 | 45 | Uterine adenomyosis | Single-channel | ||
| 16 | 46 | Uterine adenomyosis | Both | ||
| 17 | 49 | Uterine adenomyosis | Single-channel | ||
| 18 | 50 | Uterine adenomyosis | Both | OBS, HT-H | |
| 19 | 50 | Uterine adenomyosis | Both | HTN | |
| 20 | 50 | Uterine adenomyosis | Single-channel | ||
| 21 | 51 | Uterine adenomyosis | Both | ||
| 22 | 71 | Uterine adenomyosis | Both | ||
| 23 | 54 | Normal | Two-channel | ||
| 24 | 54 | Normal | Two-channel | ||
| 25 | 63 | Normal | Two-channel | ||
| 26 | 53 | Normal | Two-channel | ||
| 27 | 47 | Normal | Two-channel |
The cervical tissue samples embedded in paraffin were obtained after the surgeries or biopsies with the standard procedures,22 by pathologists from the Department of Pathology. One unstained tissue slice of 4μm thick from each patient was cut off and placed on a glass slide for FLIM measurements. After FLIM imaging, all the tissue slices were stained by H & E and examined by pathologists.
2.2. Experimental imaging setup
The fluorescence lifetime imaging for cervical tissue samples were acquired by a time-correlated single photon counting (TCSPC) system (SPC-150, Becker & Hickl, Germany) on a laser scanning confocal microscope (Olympus, FV300/IX 71, Japan) with an oil immersion objective lens (40×, NA = 1.0, Olympus, Japan). Although NAD(P)H is better excited with 350nm UV light than 405nm, there are some reports that use 405nm to simultaneously excite NAD(P)H and FAD.23,24,25 The samples were excited by a 405nm 50MHz picosecond laser (BDL-405-SMC, Becker & Hickl, Germany) with the laser power of 1–25μW. The autofluorescence signal was collected for single-channel TCSPC FLIM detection by a photomultiplier tube (PMC-100-1, Becker & Hickl) with a filter of 430nm long-pass. Alternatively, a dichroic mirror was used to spectrally split the fluorescence (Dichroic Mirror 2 as shown in Fig. 1) for two-channel TCSPC FLIM detection (Fig. 1). The fluorescence signals of NAD(P)H and FAD were simultaneously acquired by a TCSPC router (HRT-41, Becker & Hickl) and two photomultiplier tubes (PMT) with filters of 417–477nm band-pass and 508nm long-pass, respectively. NAD(P)H was detected by PMT1 (PMC-100-3, Becker & Hickl) and FAD was detected by PMT2 (PMC-100-1, Becker & Hickl). To get the photons from the two PMTs correctly separated into two channels, the delay of the system was set to 20ns for the two-channel system optimization. A fluorescence spectrum of a cervical tissue sample was shown in Fig. S1 as an example, which was excited by a 405nm continuous-wave (CW) laser. It can be seen that the fluorescence of FAD is about 6 times higher than that of NAD(P)H. In this case, the bleed-through of NAD(P)H for the FAD detection has a little impact. Therefore, a more suitable filter or excitation laser should be used for the detection of FAD signal if samples have higher NAD(P)H contributions.
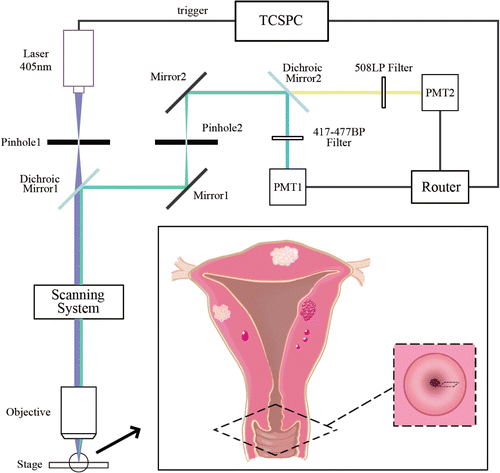
Fig. 1. Schematic of the two-channel TCSPC FLIM system for detecting benign uterine tumors though adjacent healthy cervical tissues.
Each FLIM image of 256×256 pixels was acquired in 60–120s and the area with the size of about 280μm × 280μm was imaged only once to avoid photobleaching. At least six different areas were imaged for each slide. It should be noted that the measurements were applied on the epithelium part of tissue samples, while the epithelium length in each slice is different. Some slices with longer epithelia could be measured by both single and two-channel TCSPC FLIM, but those with shorter epithelia could be measure by either single or two-channel system. As listed in Table 1, about half of patients (9 of 17) undergoing hysterectomy with uterine leiomyomas or adenomyosis were measured by both single and two-channel systems. However, the tissue samples obtained from the normal group of patients who underwent biopsies were fairly small. Only 1/10 had enough length of epithelia to be detected by both single and two-channel systems.
2.3. Data analysis
Time-decay data of each pixel in FLIM images were was fitted with multi-exponential decay models using the commercial SPCImage software (Becker & Hickl). The mask definition was applied for a region of interest (ROI) selection on the superficial and midzone layers in each FLIM image. The data is calculated from the pixels inside the ROI.
When the FLIM images were collected by the single-channel TCSPC system, triple-exponential function was used for fitting. The mean fluorescence lifetime of each pixel is calculated as tm=a1t1+a2t2+a3t3, where t1, 2, 3 is the lifetime of the fluorescent component, a1, 2, 3 is the corresponding contribution of the exponential component (a1+a2+a3=1). The tm distribution curve of the 256×256 pixels in each FLIM image was obtained by the SPCImage software, and the peak of the distribution curve was recorded.
When the FLIM images were collected by the two-channel TCSPC system, double-exponential decay analysis was applied to the FLIM images of NAD(P)H or FAD as previously reported.26 The mean fluorescence lifetime of each pixel is calculated as tm=a1t1+a2t2. For NAD(P)H, the fluorescence lifetime of free NAD(P)H solution was reported between 0.3 and 0.7ns.27,28,29 In this study, free NADH solution was measured and it was found that the fluorescence lifetime of free NADH is 0.58ns (see Fig. S2). Therefore, the value 0.5ns was set as the short-lifetime component t1 of NAD(P)H. Then, the long lifetime component t2 (bound-NAD(P)H), and their relative fractional contributions (a1 and a2) were estimated by double-exponential fitting for NAD(P)H analysis. FAD FLIM images were analysed similarly. Free FAD solution was first measured to obtain t2 of FAD as 2.9ns, and then the double-exponential fitting was used to estimate t1 of FAD, and their relative fractional contributions (a1 and a2). Based on the analysis of NAD(P)H and FAD FLIM images, the t2 of NAD(P)H and t1 of FAD distribution curves of each FLIM image was obtained and the curve peak of each image was analysed. All the goodness-of-fit χ2 values were below 1.4 indicating good fits.
3. Results and Discussion
3.1. FLIM images of cervical tissue
The cervical tissue samples were first detected by the single-channel TCSPC system with a filter of 430nm long-pass. As an example, Fig. 2 showed the autofluorescence FLIM images of an unstained slice and H & E images of the stained slice. The images exhibited the typical structure of normal cervix, which was covered by squamous epithelial cells, containing superficial, midzone and basal layers. The white dash line in Fig. 2 indicates the boundary of the basement membrane with columnar cells. Figure 2(b) displayed a capillary in the epithelium of cervix, which was composed of vascular endothelial cells. It can be seen that the FLIM images present the cellular morphology features and the characteristics of epithelium structure clearly as the traditional H & E staining method.
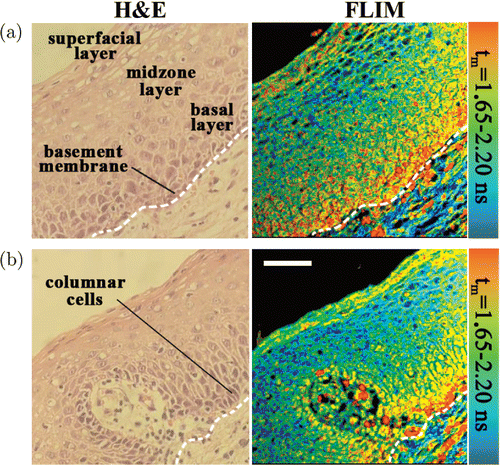
Fig. 2. H & E images and FLIM images of (a) a typical epithelium structure of normal cervix, (b) an epithelium of cervix with a capillary. The FLIM measurements were done by the single-channel TCSPC system on an unstained slide from Patient No. 8. After FLIM imaging, the slide was stained for H & E imaging. The white dash line indicates the boundary of the basement membrane. Scale bar: 50μm.
As the lifetime bar shows, the red-orange color represented short-autofluorescence lifetime, and the blue color represented long lifetime. In Fig. 3, the epithelial structure and the basement exhibited different characteristics. For a cervix tissue slide of the normal group, the fluorescence lifetime of superficial layer (Area 1 in Fig. 3(a)) was fairly short around 0.93ns, while the lifetimes increased gradually in deeper layers to around 1.73ns in Area 3. For a cervix tissue slide of uterine leiomyomas patients, the lifetime of superficial layer (Area 1 in Fig. 3(b)) was about 1.92ns and decreased to 1.38ns at the boundary of the basal layer (Area 2 in Fig. 3(b)), and that of basement across the boundary (Area 3 in Fig. 3(b)) was up to 2.03ns. For the uterine adenomyosis patients, there was no significant change at different layers of the epithelium (between Area 1 and Area 2 in Fig. 3(c)). The lifetime increased over 0.3ns in the basement (Area 3 in Fig. 3(c)) comparing with in the epithelium. It should be noted that all the images in Fig. 3 were from healthy cervixes, which had similar structures and clear distinct layers. However, the autofluorescence lifetime values showed different patterns for the normal group and benign tumor groups.
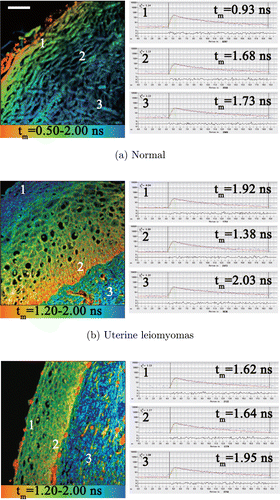
Fig. 3. FLIM images and different mean fluorescence lifetime decay curves at different areas (marked as 1, 2 and 3) of healthy cervix tissues from (a) normal (Patient No. 1), (b) uterine leiomyomas (Patient No. 9) and (c) uterine adenomyosis (Patient No. 22) patients. Area 1 and 2 was in the superficial and basal layers, respectively. Area 3 was in the basement. Scale bar: 50μm. The FLIM images were detected by the single-channel TCSPC system.
The mean fluorescence lifetime distributions of FLIM images were analysed with the triple-exponential decay model and averaged with at least six FLIM images for each patient. Twenty-two patients were divided into three groups, normal (n = 5), uterine leiomyomas (n = 8), and uterine adenomyosis (n = 9). The scatter points corresponding to the averaged lifetime for each patient were shown in Fig. 4. As shown in Fig. 4(a), the fluorescence lifetime is linearly related to patient age with a goodness of fit R2=0.999. It should be noted that the a green scatter point of Patient No. 2 was excluded for fitting. The cervical tissue of this patient (No. 2) was collected in a biopsy and measured in Nov 2017 in this work. However, the patient who underwent a operative hysteroscopy was found multiple endometrial polyps in Jan 2019. Because this data is quite different from other data, we assume that she might have endometrial polyps at the time of FLIM measurement, but it was not found in the biopsy examination. A linearly fitted line with R2=0.411 was also shown in Fig. 4(b). It can be found that the average fluorescence lifetime values of normal (black squares) and uterine leiomyomas (red circles) groups decreased with age, while those of uterine adenomyosis (blue triangles) seemed to have no correlation with age. Similar age dependence were reported for NAD(P)H and FAD redox ratio of normal cervical tissues based on spectroscopical analysis by our group7 and for NAD(P)H concentration in breast tissues by Gupta et al.30 As our group reported, the age dependence was only observed in the normal cervix group between patients aged ≤45 years and >45 years, whereas those of cervical intraepithelial neoplasia (CIN) or cervical cancer groups were indistinguishable between ≤45 and >45 years. In this study, the group of adenomyosis patients revealed similar feature as CIN and cervical cancer groups reported previously.7 Therefore, the result may imply that uterine adenomyosis growth is more invasive compared with leiomyomas, which is consistent with clinical manifestations.
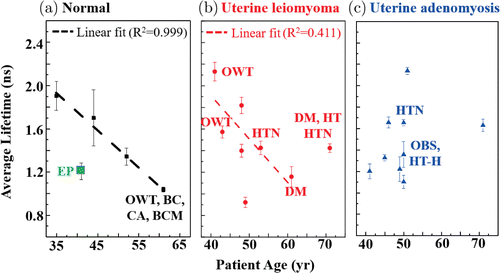
Fig. 4. Scatter plots of average fluorescence lifetime versus patient age, (a) normal, (b) uterine leiomyomas and (c) uterine adenomyosis. The dash lines in (a) and (b) were linear fitted lines. The green square in (a) was excluded for fitting, because the patient was found multiple endometrial polyps 14 months later. The patients ages, chronic diseases and medical histories are in Table 1. EP: endometrial polyps; BC: breast cancer; CA: colon adenomas; BCM: breast cancer metastasis; DM: diabetes mellitus; HT: hyperthyroidism; HT-H: hypothyroidism; HTN: hypertension; OBS: obesity; OWT: overweight. The data were from the images detected by the single-channel TCSPC system.
3.2. NAD(P)H and FAD FLIM images of cervical tissue
Considering the FLIM images (Figs. 2–4) were excited by 405nm laser and detected by the single-channel TCSPC system with a 430nm long-pass filter, the sources of fluorescence may include various endogenous fluorophores, such as NAD(P)H, FAD, collagen, elastin, and porphyrins. It is complicated to analyze the metabolism change only based on the autofluorescence with a 430nm long-pass filter. For this reason, FLIM of the two coenzymes, NAD(P)H and FAD, were simultaneously recorded by the two-channel TCSPC system with filters of 417–477nm band-pass and 508nm long-pass for further study.
Figure 5 gives an example of NAD(P)H and FAD FLIM images of a cervical tissue slide. The decay curves of FLIM images were analysed with a double-exponential model as described in Sec. 2.3. As a result, Fig. 5(a) showed the mean fluorescence lifetime tm, slow fluorescence lifetime t2, and the amplitude a2 of NAD(P)H slow fluorescence lifetime component (bound-NAD(P)H). Figure 5(b) showed the mean fluorescence lifetime tm, fast fluorescence lifetime t1, and the amplitude a1 of FAD fast fluorescence lifetime component (bound-FAD). The set of images can present the different binding states of NAD(P)H and FAD in details. The distribution curve of t2 of NAD(P)H and t1 of FAD at each image could be obtained as shown in Figs. 5(a-ii) and 5(b-ii) showing, and the peak of curves were used for statistical analysis.
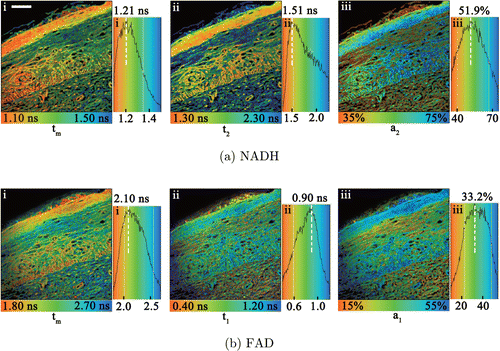
Fig. 5. (a) NAD(P)H and (b) FAD FLIM images of a cervical tissue slide and the corresponding distribution curves of the images. (i) the mean lifetime tm, (ii) the lifetime component of bound state, (iii) the fraction contribution of bound state. Scale bar: 50μm. The sample slide was from Patient No. 18. The images were detected by the two-channel TCSPC system.
3.3. Statistical analysis of NAD(P)H and FAD
For example, based on the result in Fig. 5, the peak of t2 of NAD(P)H curve (a-ii) was 1.51ns, and that of t1 of FAD (b-ii) was 0.90ns. At least six pairs of peak values of t2 of NAD(P)H and t1 of FAD were obtained for each patient, and then the average values and standard deviation of t2 of NAD(P)H versus t1 of FAD were obtained and shown in Fig. 6. A total of fifteen patients were analysed, including 6 normal, 4 uterine leiomyomas and 5 uterine adenomyosis, whose average age was 53.9±8.9 years old. The patients with age < 40 years were excluded owing to the limited number of young patients with adenomyosis or leiomyomas. In addition, the patients with age < 40 years may have different metabolic status. The 15 patients were marked their numbers, chronic diseases and medical histories in Fig. 6. Their ages and other major medical informations were also listed in Table 1.
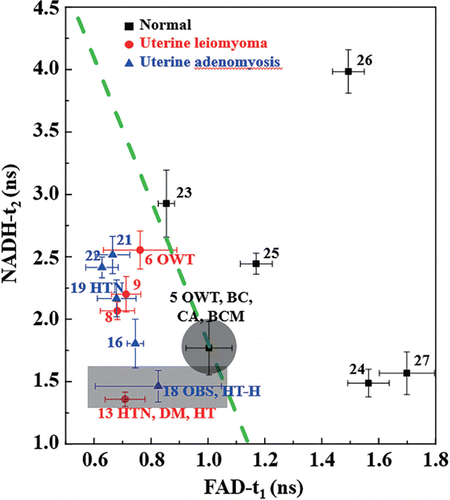
Fig. 6. Differentiating benign uterine tumors from healthy uterine group by studying the cervix tissues. The plot t2 of NAD(P)H versus t1 of FAD was obtained from 15 patients, whose ages, chronic diseases and medical histories were in Table 1. BC: breast cancer; CA: colon adenomas; BCM: breast cancer metastasis; DM: diabetes mellitus; HT: hyperthyroidism; HT-H: hypothyroidism; HTN: hypertension; OBS: obesity; OWT: overweight.
It was found that the healthy uterine group (n=6) and the group of benign uterine tumors (n=9) were in two different areas (Fig. 6). All of the benign uterine tumor patients were in the lower left. Most of the healthy uterine group (5 of 6) were in the upper right. The separating line (y=−5.70x+7.51, green dash line in Fig. 6) was performed using the linear discriminant analysis with the accuracy of 92.1%. It should be specially noted that Patient No. 5 is in the healthy uterine group, who has a long surgery history. This patient underwent modified radical mastectomy for breast cancer, partial colectomy for colon adenomas, cholecystectomy, and lumpectomy for breast cancer metastasis during the past ten years. It is reasonable to hypothesize that the metabolic status of Patient No. 5 with a malignant tumor history is extraordinarily different from others of the healthy uterine group. Therefore, the result suggests that FLIM might be useful for the discrimination of healthy uterine and benign uterine tumor by detecting the fluorescence lifetime of NAD(P)H and FAD from the adjacent cervical tissues.
3.4. Effect of chronic diseases on metabolism
It is well known that there are some chronic diseases such as diabetes, hyperthyroidism, hypertension, and obesity, associating with metabolism problems or affecting metabolism level.31,32 Patients Nos. 13 and 18 highlighted in Fig. 6 showed much lower t2 of NAD(P)H than others. Patient No. 13 was diagnosed with diabetes, hyperthyroidism, and hypertension. Patient No. 18 has hypothyroidism and obesity. For a comparison, Patient No. 19 has only hypertension, whose data showed little difference than others. It may indicate that there is a correlation between the chronic diseases and metabolically abnormal environment, leading to lower t2 of NAD(P)H. For Patient No. 6, who is an overweight patient, her data was in the area of the lower left, but showed larger standard deviation on t1 of FAD than others. Moreover, Patient No. 18 with hypothyroidism and obesity showed much larger standard deviation on t1 of FAD. The larger variability of one patient may indicate the variation of cellular metabolism and microenvironment became larger with weight gain progression. Further investigation is needed with a larger number of cases.
4. Conclusion
NAD(P)H and FAD are important coenzymes in cellular energy metabolism. The FLIM measurements of NAD(P)H and FAD in the adjacent healthy cervical tissue may be useful for detecting the benign uterine tumors such as leiomyomas and adenomyosis. This method proposed a novel strategy for detecting uterine leiomyomas and adenomyosis to avoid hysterectomy by measuring the adjacent healthy cervical tissues. It was also found that ages, diabetes, hyperthyroidism, and obesity could affect metabolic level and environment, as well as a malignant tumor history.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (NSFC) (Grant Nos. 11574056, 61575046 and 11804350), Ministry of science and technology of the People’s Republic of China (China-Serbia bilateral project SINO-SERBIA2018002), the Shanghai Sailing Program (Grant No. 17YF1421300), Fudan University-CIOMP Joint Fund (FC2017-007, FC2018-001), and Pioneering Project of Academy for Engineering and Technology, Fudan University (gyy2018-001, gyy2018-002). We also thank all the patients involved in this study.
Maojia Huang and Zixiao Zhang contributed equally to this study.