Enhancement of microvessel in laser speckle image using gaussian kernel template
Abstract
Laser speckle contrast imaging (LSCI) is an optical imaging method, which can monitor microvascular flow variation directly without addition of any ectogenous dye. All the existing laser speckle contrast analysis (LASCA) methods are a combination of spatial and temporal statistics. In this study, we have proposed a new method, Gaussian kernel laser speckle contrast analysis (gLASCA), which processes the raw images primarily with the Gaussian kernel operator along the spatial direction of blood flow. We explored the properties of gLASCA in the simulation and animal cerebral ischemia perfusion model. Compared with the other existing speckle processing methods based on spatial, temporal, spatial-temporal or anisotropic linear structure; the present gLASCA method has a high spatial-temporal resolution to respond the change of velocity especially in microvasculature. Besides, the gLASCA method obtains approximately 10.2% and 7.1% higher contrast-to-noise ratio (CNR) over the anisotropic linear method (aLASCA) in the simulation and experiment models. For these advantages, gLASCA could be a better method for local microvascular laser speckle imaging in terms of cerebral ischemia reperfusion, spreading depression and brain injury diseases.
1. Introduction
In recent years, laser speckle contrast imaging (LSCI) has become an emerging method of optical vascular flow imaging.1 Compared with brain functional imaging methods, such as near infrared spectroscopy imaging and laser Doppler, LSCI has a higher temporal resolution. Moreover, LSCI is a non-invasive method without the introduction of any exogenous dyes in consideration of angiography and fluorescence imaging.2 LSCI can simultaneously monitor the cerebral cortex of vascular morphology and blood flow velocity.3 At present, the contrast value () in laser speckle contrast analysis (LASCA) is used to study capillary blood flow,4 retinal blood flow5 and cerebral blood flow (CBF).6,7
Scattering laser reflected from a rough surface with random interference leads to the generation of speckle pattern, which is spatially blurred due to the movement of scattering particles. Definition of speckle contrast in a local region can be expressed as the quotient of the standard deviation value to the mean value of the light intensity, . Traditionally, is calculated in the spatial domain of laser speckle spatial contrast analysis (LSSCA)8 or the temporal domain of laser speckle temporal contrast analysis (LSTCA).9 The spatial resolution and temporal resolution are compromising with each other. Many studies have reported that the isotropic approach could address the issue of spatiotemporal resolution.
Spatially derived contrast using LASCA (sLASCA) proposed by Briers and Webster,10 is an enhancement of the basic LSSCA technique. In sLASCA scheme, the derived contrast value can be obtained by averaging over temporal frames of raw speckle images, which is different from LSSCA. Temporally derived contrast using LASCA (tLASCA) also focuses on the statistical temporal frames.11 For example, in tLASCA method, the parameter means the number of temporal frames, usually the total convolutional window selected is (3)(3)(/2) where pixel is the spatial window. Both methods would smooth LSSCA or LSTCA images in the spatial or temporal domains to obtain the degree of robustness. Furthermore, in spatial-temporal cuboid LASCA (stLASCA)12 method, a cuboid of pixels can be used to calculate the local for achieving the flexible and quantitative scheme in spatiotemporal domain. Since speckle blurring of blood flow can show directional sensitivity, anisotropic LASCA13 (aLASCA) proposed by Abhishek focuses on the anisotropic spatial neighborhood and calculates linearly local speckle contrast. aLASCA not only improved microvascular distinguishability, but also strengthened the local noise.
Considering the advantages and flaws in all the methods, we introduced a Gaussian kernel LASCA (gLASCA) method of matched operator to acquire the local speckle contrast along the estimated vascular direction. Then, we performed the simulation and mice experiments to investigate and assess the performance of this algorithm. Comparing with the other existing speckle processing methods based on spatial, temporal, spatial-temporal and anisotropic linear structure, the present method improved the contrast-to-noise ratio and had a high spatial-temporal resolution in microvasculature. Meanwhile, the matched operator in gLASCA method also decreased the local noise and smoothed the main branches of blood flow in processed images.
2. Methods and Experiment
2.1. Simulation of time integrated dynamic speckle image
The time-integrated dynamic speckle images from “copular” theory14 is described as Eq. (1).
2.2. Experimental setup
Figure 1 shows the schematic diagram of the LSCI system. A He-Ne laser (632.8nm and 3mW, SPL-HN250R, Hangzhou SPL Photonics, China) is adjusted by a collimator. Then the laser passes an expander and irradiates the imaging region with a diameter of 12mm and the incident angle of 30–45∘. The beam expander works at 450–680nm. Meanwhile, the image of illuminated region is magnified by a zoom stereo microscope (50486A, Navitar, USA) and captured by a monochrome 12 bit CCD camera (GS3-U3-51S5M-C, Point Grey, Canada, 3.45m per pixel) with pixels. The exposure time of the CCD was 15ms. Images are acquired through the programming software at 20Hz. Then, a stack of 30 raw images is stored and processed using MATLAB routines (Mathworks, MA, US).
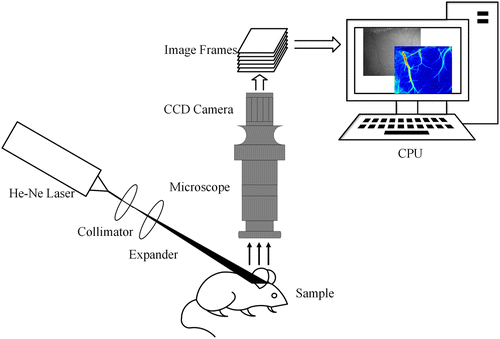
Fig. 1. Schematic of LSCI system.
2.3. Animal preparation
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Nanjing University of Aeronautics and Astronautics. The Institute of Cancer Research (ICR) mice were purchased from the Qinglongshan Animal Experiment Center (Nanjing, China) and housed in a cage for 7 days after individualization (postnatal day [PD] 36).
Five ICR mice were anesthetized by using 5% chloral hydrate (400mg/kg, i.p.) and placed in a stereotaxic frame with skull-reduced flat orientation. The skin was removed and a cranial window (about 2mm 2.5mm) was drilled right side of midline overlaying the parietal cortex. The imaging acquisition was performed on the cranial window after the end of the surgery and temperature was continuously measured.
In this study, a transient middle cerebral artery (MCA) occlusion model was induced as reported protocol.16 A small micro-clip was placed tenderly on the most proximal branch of one side of the distal carotid arteries. After 20min, the micro-clip was removed and both carotid sutures were released to allow for reperfusion. Body temperature was maintained at 37.8∘C by a heat lamp during surgery and for 2h after the start of reperfusion.
2.4. Design of matched operator
Microvessels had several small curves. The anti-parallel pairs could be approximated by piecewise linear segments.17,18 We utilized a more effective method and selected the suitable line operator.16 The minimal value of contrast sum is defined as Eq. (2)
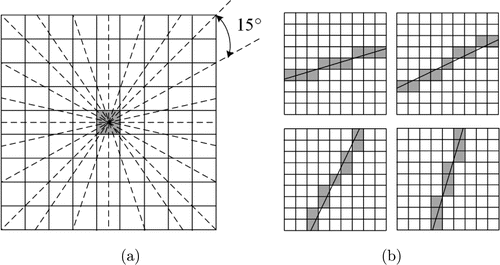
Fig. 2. (a) 12 directions to assess line intensity of shaded pixel. (b) lines of 9 pixels with direction: 15∘ (top left), 30∘ (top right), 60∘ (bottom left), and 75∘ (bottom right).
Furthermore, the vascular width decreased while it travelled radially outward from main vein and aorta, such variation of vessel caliber was taper, which might be estimated by a Gaussian curve.19 When the definition of matched filter is extended to two-dimensional images, the convolution kernel will fluctuate by subtracting the average of Gaussian curve from the literature.20 This kernel could be mathematically expressed as Eq. (3).
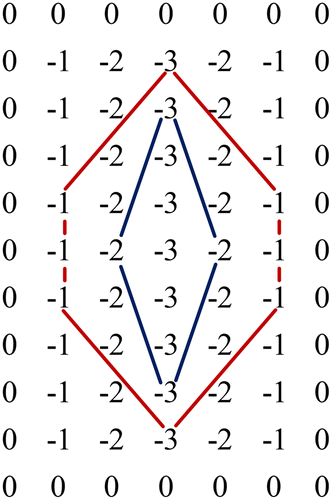
Fig. 3. The Gauss kernel that has been to match the segment along the vessel direction. Here the region blue line circled corresponds to a spatial window and the red line circled corresponds to a spatial window.
For the vessels at different orientations, the kernel should be rotated accordingly. The rotation matrix is given by Eq. (4).
If denotes the value in the window of Gaussian kernel, the weight coefficient () of Gaussian kernel function is described as Eq. (5).
3. Results
gLASCA showed a better performance for in vivo imaging of simulated dynamic speckle images comparing with other five representative processing methods including LSSCA, LSTCA, tLASCA, sLASCA, and stLASCA. Table 1 shows the neighborhood parameters in each algorithm. The spatial and temporal resolution were the key factors for choosing appropriate parameters (Table 1). Both the processing frames of simulation and experiments speckle images were 30 frames in different conditions except in LSSCA method. Thus, we selected 5 frames (Nt) as temporal convolution window aimed at the spatial-temporal convolution algorithms. Additionally, Ns shown in Table 1 ensured a fair comparison among these 6 algorithms. Since neither spatial resolution of speckle images in simulation model nor mouse experiment was identical, Ns need to be selected on the basis of pixel size, which means that the higher image resolution would correspond to the larger spatial window (Ns) except for LSTCA method.23
| Processing method | Spatial convolution window (Ns) (simulation/mouse) | Temporal frames () (simulation/mouse) | Temporal convolution window (Nt) (simulation/mouse) |
|---|---|---|---|
| LSSCA | / | 1/1 | NA/NA |
| LSTCA | / | 30/30 | NA/NA |
| tLASCA | / | 30/30 | (N/2)/(N/2) |
| sLASCA | / | 30/30 | NA/NA |
| stLASCA | / | 30/30 | 5/5 |
| aLASCA | / | 30/30 | 5/5 |
| gLASCA | Linear operator (same counts) | 30/30 | 5/5 |
The contrast images with LSSCA, LSTCA, tLASCA, sLASCA, stLASCA, aLASCA and gLASCA methods for the simulated data were shown in Figs. 4(c)–4(i). We hypothesized that the intensity image shown in Fig. 4(a) of scattering surface was cerebrovascular image where dark regions of radial shape represented the blood vessels. Arbitrarily, the intensity of radial line region was 0.5 and the background region was 0.9. As mentioned above, the parameter in Eq. (1) affected the correlation time . Therefore, we simulated a series of flow velocity of dynamic speckle images by changing parameter from 5 to 25 in the radial line region (30 frames). For example, the speckle image of was shown in Fig. 4(b) and the contrast images of and were shown in Figs. 4(c)–4(i). The intensity of radial line region showed higher value in the first row () than that in the second row (), which illustrated that a smaller corresponds to a smaller 1/, as well as a faster spatial blurring of the speckle pattern. gLASCA method also complied with this theory. Besides, Fig. 4(h) showed more spurs than Fig. 4(i) along the edge of radial lines.
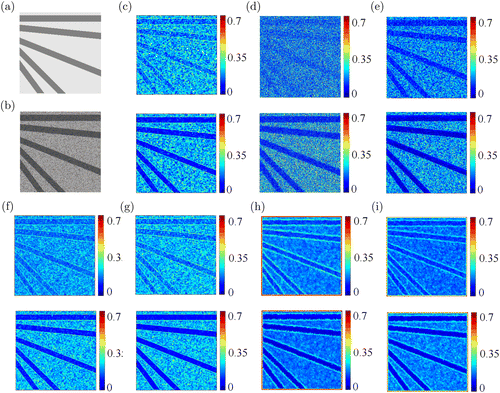
Fig. 4. (a) Intensity image of scattering surface. Dark region represent blood vessels and the gray value is constant. (b) Simulated time-integrated dynamic speckle images with “copular” theory. Comparisons between contrast images computed from the simulated speckle images with parameter using (c) LSSCA, (d) LSTCA, (e) tLASCA, (f) sLASCA, (g) stLASCA, (h) aLASCA, (i) gLASCA methods (top: ; bottom: ).
The contrast images calculated from mice brain vessels were shown in Fig. 5. The field of view was approximately mm. The size of each raw speckle image was pixels. According to the principle of the LSCI, the resolution of the image was 4.88m. gLASCA produced a significant improvement in micro vessel distinguishability of high resolution especially under the condition where vessels has a low flow velocity in red square ROIs of Fig. 5. These microvessels were indistinguishable in both LSSCA and LSTCA images shown in Figs. 5(a) and 5(b). Meanwhile, those images calculated using spatiotemporal methods shown in Figs. 5(c)–5(e) show vague and small graininess. The vessels were not like the continuous lines, but rather as sprays of dots. Compared with the aLASCA, gLASCA image displayed better contrast between micro vessels and tissues.
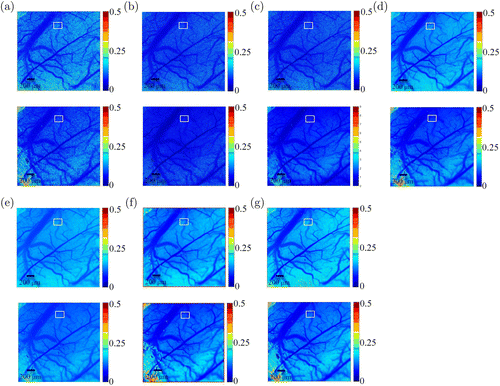
Fig. 5. Comparisons between contrast images computed from mice brain vessels using (a) LSSCA, (b) LSTCA, (c) tLASCA, (d) sLASCA, (e) stLASCA, (f) aLASCA, (g) gLASCA methods (top: ; bottom: ).
Furthermore, the global contrast value using spatial methods was higher than that by using temporal methods in CBF images, which was similar in the simulation of blood images. Interestingly, we also witnessed that the intensity of cerebral vascular region was higher in the first row (min) than that in the second row (), which illustrated the dynamic variation of cortical blood flow pre- and pose the reference point was in accordance with that of the simulation model. In other words, the increasing parameter was associated with the fast-moving particles, so it directly showed the CBF increased after the reference node of perfusion.
In Figs. 6(a) and 6(b), we calculated all the mean contrast values of 25 regions of interest (ROI), such as the ones highlighted by rectangle in Fig. 5, in vascular region with methods of LSSCA, LSTCA, tLASCA, sLASCA, stLASCA and gLASCA. In Fig. 6(a), all the mean contrast values kept a fall trend when the parameter increased gradually with the interval of 5. While the mean contrast value increased slowly before reference point and dropped substantially after reference node in animal perfusion models, notably the time point 20 was the reference point of the beginning of perfusion shown in Fig. 6(b). The tendency of speckle contrast became highly homogeneous in simulation and experimental mouse model. We could observe that the mean contrast value obtained by stLASCA was the largest among the others.
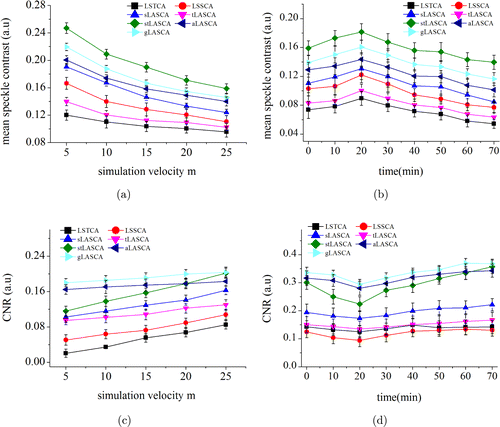
Fig. 6. Speckle contrast analysis of simulated dynamic speckle and mice cerebral blood vessels. Changes of (a) mean speckle contrast and (c) contrast to noise (CNR) with in Eq. (1) by using 6 algorithms. Changes of (b) mean speckle contrast and (d) contrast to noise (CNR) pre- and pose the typical local perfusion by using 6 algorithms. (notably the time point 20 is the reference node of the beginning of perfusion).
Meanwhile, we calculated the CNR of 25 ROIs to evaluate the quality of LSCI images with different algorithms. The value of CNR was defined as a distinguishability between the CBF and the background. We found that gLASCA method showed best accuracy in representing flow velocity levels of distinguishable and lowest noise in simulation and experiment models in Figs. 6(c) and 6(d). gLASCA method showed 10.2% (, test) and 7.1% (, test) higher contrast-to-noise ratio (CNR) both in the simulation and experiment models compared to aLASCA.
Figure 7 represented the CBF image of the cranial window of cortex for the mice, before and after the perfusion. We noted that the CBF change of pos-perfusion changes circled by gray rectangle were substantially higher than that of pre-perfusion, which was consistent with variation of the tissue background in blue rectangular box. Moreover, we could find a surge pos-20 min at the reference point, which was in accordance with transient cerebral ischemia reperfusion physiological mechanism shown in Fig. 7(i). Therefore, it is very important to monitor the microcirculation tissue perfusion.
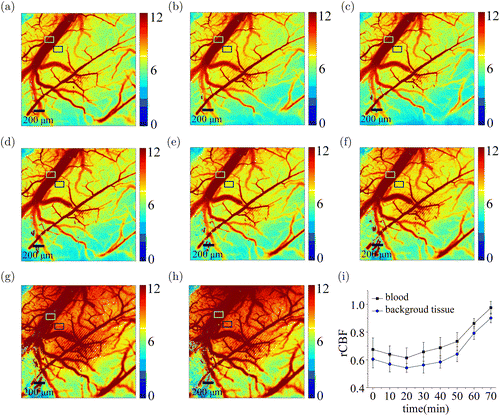
Fig. 7. Relative CBF changes of laser speckle images of local perfusion mice at each time point including (a),(b), (c), (d) (e), (f),(g) and (h), notably the time point (c) is the reference node of the beginning of perfusion. (i) Normalized relative CBF and background tissue of the ROI calculated using LLSCI.
gLASCA is an algorithm to process speckle data preferentially along the flow direction. In micro-vessels, the direction of minimum contrast is along radially the blood vessels. Therefore, we can obtain the direction of blood flow as long as the direction of speckle contrast selected is the minimum. Thus, we proved that the present gLASCA algorithm brain had a significant resolution improvement in microvasculature over current techniques imaging of mouse.
4. Discussion
In the present study, the method of gLASCA processed laser speckle image with the preferred convolutional mark, which aligns with the vascular flow direction. This algorithm just required three raw spectral image frames for contrast calculation, which provided a superiority in temporal resolution of cerebrovascular imaging. Compared with other spatiotemporal algorithms, the contrast images obtained by using gLASCA method showed a margin increase in CNR of ROIs.
Since segments kernel selected along the direction of each 15∘ angle were found to respond well, 12 different templates were applied in the our proposed method to search for the optimal direction operator along all possible orientations.24 Thus, applications of the designed Gaussian operator enhanced value discrepancy in the anisotropic method based on speckle image. Meanwhile, a proper convolutional mark along the direction of the minimal value could be applied in refresh a high-quality contrast image in anistropic method, especially in the condition of slower flow velocity as shown in Figs. 4 and 5. Selecting parameter as a smaller value would match well with cerebral microvessels of medium caliber for the cerebral images acquired by our experiment equipment.25
Unlike LSTCA with the characteristics of highest spatial pixel resolution, all other spatial or spatiotemporal algorithms sacrifices the spatial resolution with a minimum of pixels in simulation models, as well as pixels in experiment models. Of note, gLASCA has a spatial resolution of Gaussian matched operator where the mask is the resolution match the vessel axis shape. Furthermore, gLASCA has a better performance in contrast with the other algorithms to assess the relative changes for CNR. We observe that the spatial algorithm reacts better to the static object of the scattered source, whereas the temporal algorithm reacts more promptly to the dynamic object of the scattered source.26 In clinical trials, blood flow in human tissue would change over time, so did laser speckle image system. Thus, our algorithm can adapt itself to improve accuracy along the orientation of blood flow.
We have explored the superiority and feasibility of gLASCA to employ in mice perfusion studies. Despite pulsatility of blood flow induced by heart rate should keep the frequency of 2.25Hz to 3Hz, tLASCA just required a temporal window of 40 frames in total of 1.6s, given the camera speed (45 frames/s). Therefore, gLASCA with the temporal convolution window can apply in less frames per second to monitor the perfusion response. It is a recommended technique for scientists to research the animal models of cerebrovascular reactivity.
Acknowledgments
This study was supported by National Major Scientific Instruments and Equipments Development Project Funded by National Natural Science Foundation of China (81827803, 81727804), National Natural Science Foundation of China (61875085, 81601532), Natural Science Foundation of Jiangsu Province (BK20160814), Jiangsu Science and Technology Support Plan (Social Development) (BE2016759), and Jiangsu Innovation Program for Graduate Education (KYLX16_339). All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.