Combined spatial frequency spectroscopy analysis with visible resonance Raman for optical biopsy of human brain metastases of lung cancers
Abstract
The purpose of this study is to examine optical spatial frequency spectroscopy analysis (SFSA) combined with visible resonance Raman (VRR) spectroscopic method, for the first time, to discriminate human brain metastases of lung cancers adenocarcinoma (ADC) and squamous cell carcinoma (SCC) from normal tissues. A total of 31 label-free micrographic images of three types of brain tissues were obtained using a confocal micro-Raman spectroscopic system. VRR spectra of the corresponding samples were synchronously collected using excitation wavelength of 532nm from the same sites of the tissues. Using SFSA method, the difference in the randomness of spatial frequency structures in the micrograph images was analyzed using Gaussian function fitting. The standard deviations, σ calculated from the spatial frequencies of the micrograph images were then analyzed using support vector machine (SVM) classifier. The key VRR biomolecular fingerprints of carotenoids, tryptophan, amide II, lipids and proteins (methylene/methyl groups) were also analyzed using SVM classifier. All three types of brain tissues were identified with high accuracy in the two approaches with high correlation. The results show that SFSA–VRR can potentially be a dual-modal method to provide new criteria for identifying the three types of human brain tissues, which are on-site, real-time and label-free and may improve the accuracy of brain biopsy.
1. Introduction
Human brain tumors appear regardless of color and age. Brain tumors may arise from a brain’s internal tissue (primary brain and central nerve system (CNS) tumors) or external metastases to the brain (metastatic brain tumors). Nearly 700,000 people in 2018 were living with a primary brain and CNS tumor in the USA.1
The primary origin of brain cancer is brain neoplasm cells. The secondary or metastatic brain tumors, which have spread to the brain or cerebellum from another location (organ) in the body, are much more common than primary brain tumors. They are the most common brain tumors in adults. Metastatic brain tumors make up about one-fourth of all cancers that spread through the other organs of body. The cerebellum is the part of the brain that watches over complex muscle movements. About 15% of metastatic brain tumors develop in cerebellum, while about 85% of metastatic lesions are located in the cerebrum. The cancers spread to the brain are commonly from lung, breast, colon, kidney, melanoma, thyroid and uterine. The cancer cells of these organs broke free of primary cancer, travel through blood and spread to the brain and grow into the new tumors. The brain metastases of lung cancer are the most common forms of metastatic brain tumor. In fact, lung cancer staging is often found in brain lesion scan before the primary lesion in lung. Approximately 173,770 cases of lung cancer were diagnosed in the US in 2004 with an estimated 160,440 deaths.2 Small cell lung cancer (SCLC) accounts for approximately 20% of all cases of lung cancers.3 It tends to disseminate earlier during its natural history than non-SCLC (NSCLC) and is clinically more aggressive.4
The conventional diagnosis of brain lesions is performed using biopsy and histopathology. This gold standard method requires freezing specimen or reagent preparation for the biopsy tissue prior to microscopic analysis. This requires skilled technicians and histopathologic experts to perform the diagnosis as well as considerable time before results become available. The subjective nature of the histological evaluation is associated with considerable intra- and inter-observer variation in grading the biopsy tissues, which strongly depends on the experience of the medical doctors. Magnetic resonance imaging (MRI) provides preoperative information about the localization, size and molecular-level biochemical components of the tumor. However, some information is lost during surgery due to tissue changes, e.g., profile shifts, which is the largest concern of the surgeons. In addition, MRI and computed tomography (CT) are expensive non situ detection methods that have several disadvantages, such as time-consuming and inflexible.5,6 Therefore, it is of importance to develop an objective and noninvasive method that can be used for precancerous diagnosis and can be used during clinical practice of surgery.
Optical spectroscopic techniques (e.g., Raman and fluorescence) have demonstrated their advantages of being real-time, in situ and label-free in biomedical applications. Since the first report of optical spectroscopic technology used to differentiate human lesions from normal tissues,7,8,9 optical spectroscopic techniques have become a major thrust as a potential noninvasive tool to detect changes in the state of tissues and obtain molecular information. Among them, Raman spectroscopy has been widely used for diagnostic purpose. Recently, native biomarker using Raman spectroscopy was reported in studies of aggressive brain cancers.10,11,12,13,14,15,16
A visible resonance Raman (VRR) spectroscopy method, in recent years, was shown to have advantages over the conventional Raman technology due to the following reasons: (1) VRR has some unique features. For instance, more resonance Raman (RR) intrinsic biomarkers were observed that have been generated from multiple vibrational modes of larger biomolecules due to resonance Raman using visible 532nm excitation which matches many biomolecular electronic transition levels in tissues, cells and organelles. (2) VRR signal intensity may be enhanced 102 to 103 times while operating in situ in a time period of seconds. (3) VRR-enhanced signal from vibrational chemical bonds of molecules can be detected at molecular concentrations less than 1.0nM and the activity of particular molecular species can be targeted preferentially. VRR provides an effective way to enhance Raman signal from particular chemical bonds associated with key molecules and detect their changes at a molecular level, such as carotenoids, tryptophan, amides, lipids and protein in breast, skin, heart, gynecologic and brain lesions.14,15,17 These advantages of VRR have led to a rapid progress in its applications in brain and other human cancer diagnosis14,17,18,19,20,21 that are difficult to achieve by conventional nonresonance Raman method.22,23,24,25,26,27
Recently, another new optical spatial frequency spectroscopy analysis (SFSA) method was introduced based on Fourier spatial frequency spectrum analysis of the underlying structure pattern of tissues. The spatial frequency of the structure has been used to analyze microscopic images of cervical cancer at different grades to obtain the information of the periodic and random structures of the tissues.28,29,30 The images of certain types of tissue sample contain intensity patterns of the underlying structures, which can be considered as a composition of different spatial frequencies. Spatial frequency spectral analysis method can be used to convert the spatial structure of recorded optical intensities in the images into spatial frequency spectra, analogous to the Fourier decomposition of color frequency spectra with both being frequency-domain analysis.31 Most importantly, the result of SFSA is a function of the underlying structure of the tissue and can therefore give a truly objective picture of pathology.
This study focuses on analyzing the micrograph images and VRR spectra to find key markers to differentiate human brain metastasis of lung cancer including adenocarcinoma (ADC) and squamous cell carcinoma (SCC) from normal tissues using dual optical modalities of SFSA–VRR, synchronously, in situ, ex vivo, in real time. The criteria for identification of the three types of brain tissues will be created using SFSA–VRR method. The SFSA–VRR method may have potential for new applications in neuropathology diagnosis and brain surgery.
2. Materials and Methods
Specimens: Human tissues of brain metastases of lung cancer and normal brain tissues were obtained from the Air Force Medical Center, PLA, Beijing, China. All the specimens of brain metastases of lung cancer tissues were carcinomas. Two major subtypes of brain metastasis from lung cancer were used including ADC and SCC. ADC specimens from five patients and SCC specimens from three patients and one normal brain tissue specimen from a noncancer carrying patient were diagnosed. The histopathology reports of metastatic brain tumor and normal brain tissues based on standard pathology and immunohistochemistry methods were obtained for each specimen. According to the stage classification, all investigated tumors are distant cancer metastases with stage IV (The international standard for classification and nomenclature of histology is the International Classification of Diseases for Oncology-ICD-O-3).32 The experimental procedures were approved by the committee of the Air Force Medical Center, PLA, Beijing, China.
Micrograph images: 30 micrograph images of metastatic brain tumors and normal tissues (4 from normal tissue, 26 from cancerous tissues) were recorded as bright-field reflectance images with white light illumination. Typical micrograph images are shown in Figs. 1(a)–1(c). All images were taken under identical conditions. The micrograph images were obtained label-free using the confocal Raman microspectroscopic system, i.e., Jobin Yvon confocal micro-Raman spectrometer (JY-HR-800 France) with image resolution 500μm×380μm, and image size 538 pixel×403 pixels. The micrograph images were recorded before and after VRR data collection in order to confirm the stability and repeatability of the micrograph images.
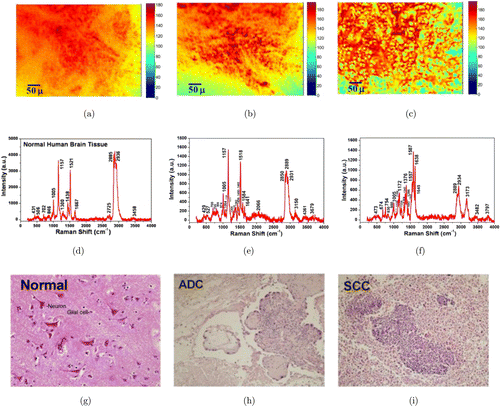
Fig. 1. Three sets of data from brain metastases of lung cancer and normal tissue. Upper row: A set of typical cropped confocal micrograph images using pseudo color of (a) normal brain tissue, (b) brain metastasis from ADC, and (c) brain metastasis from SCC. Middle row: A set of RR spectra of (d) normal brain tissue, (e) brain metastasis from ADC and (f) brain metastasis from SCC. The spectra in (d)–(f) are corresponding to the images in (a)–(c), respectively. Bottom row: H&E-stained images from (g) normal brain tissue, (h) brain metastasis from ADC and (i) brain metastasis from SCC. All the H&E images used 20×objective and correspond to the images in (a)–(c), respectively.
VRR spectral measurements and Raman instrument: 30 VRR spectra were recorded from the same sites of the specimens where the micrograph images were acquired synchronously. Each brain cancerous tissue was identified by traditional pathology using H&E (hematoxylin and eosin) and immunohistochemical assay as the gold standard (Figs. 1(g)–1(i)). VRR spectra were acquired using confocal micro-HR800 Raman spectrometer with 532nm excitation wavelength. The detailed description of the instrument was previously reported.10,18 The excitation light beam was directly shed on the surface of the samples. The spot diameter of the focused laser beam at the sample position is about 1μm. The laser power at the sample position was 0.9mW and the typical integration time was 30s. All spectra were acquired at room temperature. The final spectral resolution was 2cm−1 in the range of interest from 200 to 4000cm−1. The micrograph images and VRR spectra were then analyzed and compared to the pathology and immunohistochemistry reports.18
The basic principle of SFSA: In this paper, the micrographic images (Figs. 1(a)–1(c)) from human brain cancer and normal tissues ex vivo, differ from the pathology biopsy images (Figs. 1(g)–1(i)), and were analyzed using SFSA method as described in Refs. 28–30. The current study focuses on human brain metastases of lung cancer. Usually, the intensity distribution of the micrographic image can be expressed as Fourier Transform (FT)31:
Implementation of SFSA: Images of human normal brain tissues and brain tissues of metastases from lung cancers obtained from confocal micrograph were analyzed using Fast FT (FFT) to generate the spatial frequency spectra. Further processing of the spatial frequency spectra allows the extraction of underlying information of the tissue structure.
3. Results and Discussion
Identification of human normal brain tissues and brain tissues of metastases from lung cancer by VRR (1): Three sets of data collected from representative brain metastases of lung cancers (ADC and SCC) and normal tissues are displayed in Fig. 1, including (a) the micrograph images (top row), (b) the VRR spectral data measured synchronously with the micrograph images (middle row) and (c) microscopic images for histopathology diagnosis with H&E stains (bottom row).
VRR spectral fingerprints of carotenoids for ADC and SCC (1): The typical three types of VRR spectra from normal human brain tissues and brain metastases of lung cancers (ADC and SCC) are shown in Figs. 1(d)–1(f), respectively. VRR spectra of key fingerprints from healthy brain tissues in Fig. 1(d) display eight major peaks at 1005, 1157, 1521, 1438, 1667, 2850, 2885, 2932cm−1, respectively. The relatively sharp and enhanced peaks of 1157 and 1521cm−1 are supposed to arise from carotenoids, low-density lipoprotein (LDL) and high-density lipoprotein (HDL). RR peaks at 1438, 2850 and 2885cm−1 for rich fatty acids are stronger than protein bands at 1005, 1667 and 2934cm−1 in peak intensities. The resonance-enhanced intrinsic molecular fingerprints are supposed to mainly arise from carotenoids with intense RR modes at 1157cm−1 (νs(C–C)), 1521cm−1 (νs(C=C)) and 1008cm−1 (ρ(C– H3), ν(C–C)) and major overtone peaks at 2320 and 2667cm−1. Carotenoids play a significant role in normal brain tissues33,34,35,36,37,38,39,40,41 and act against the destructive effect of the free radicals. The specific importance of carotenoids is to serve as a marker substance for the entire anti-oxidative network of human brain tissues.42,43
In contrast, in brain cancers, peaks of carotenoids at 1161 and 1521cm−1 are occurring but obviously weaker for ADC and significantly lower or tend to disappear for SCC compared with normal tissue. We proposed that the dependence of the RR spectra of biomarker carotenoids on the tissue types (ADC and SCC) revealed the difference between the types and the status of each type. Our measurements found that these two resonance bands of 1157 (1161) cm−1 and 1521cm−1 are active because carotenoids have a pre-resonance absorption band around 480 nm and the excitation wavelength of 532nm falls in this pre-resonance range. The intensities of RR peaks of 1157 and 1521cm−1 decreased as the type of brain cancer changes from normal to cancerous. This may indicate a progression of different mutation processes. The RR peaks of 1157 and 1521cm−1 diminish sharply for SCC brain tissues (Fig. 1(f)). The suggested reason was that the content of carotenoids decreased, which caused the RR peaks to become too weak to detect or induced a shift in chemical vibration bands. This may be due to the structural changes within the microenvironment of malignancy. Carotenoids are natural fat-soluble pigments with the reduction of fat. For instance, the peak 1521cm−1 drastically decreases with the decreasing content of saturated fatty acid lipids (1442 and 2850cm−1). All these observations indicate the existence of a fast activation and deactivation of Raman vibrational modes at 1521 and 1157cm−1 in the types of ADC and SCC brain tissues.18 RR modes of 1157 and 1521cm−1 could be a significant marker to diagnose metastatic brain cancerous tissues and other cancers.44,45
VRR spectral fingerprints of tryptophan for ADC and SCC (2): The key fingerprint of tryptophan molecules with a main vibrational mode at 1587cm−1 (W8b) was observed in the RR spectra.46,47,48 It was found that endogenous tryptophan and metabolites contributions were accumulated in brain metastases of lung cancer tissues, and the RR vibrational mode at 1584–1587cm−1 in malignant ADC and SCC (Figs. 1(e) and 1(f)) was enhanced in intensities by resonance. Researchers have reported that heterocyclic amino acid tryptophan is a key factor during the metabolic process in human brain tissues.49,50,51,52,53,54 This RR vibrational mode of 1587cm−1 indicates that tryptophan is produced in human metastatic brain tumor of lung cancer and the kynurenine pathway of tryptophan metabolism is involved in brain tumor progression.46 These observations are in good agreement with changes of tryptophan contents in human brain malignant meningioma and gliomas tissues10 and skin basal cell carcinoma tissues.19 The molecular biomarker of tryptophan provides a potential approach to diagnose metastatic brain cancerous tissues and other cancers.
VRR spectral fingerprints of lipid and protein for ADC and SCC (3): The VRR peaks of protein (2934cm−1) gradually increased in different types of metastatic brain tumors of lung cancer (ADC and SCC) in comparison with normal brain tissues shown in Figs. 1(d)–1(f). The RR mode of 2885cm−1 (2850cm−1) is correlated with the lipid content and fatty acids of lipids which have intense contributions in normal bran tissues. The increase in the intensity of methyl (–CH3) and decrease in the intensity of methylene (–CH2–) (protein and lipid, both showing symmetric stretch modes of 2934 and 2850cm−1, 2885cm−1) were observed in both types of metastatic brain tumor tissues of ADC and SCC. The ratio of RR peaks of protein to lipid (I2934cm−1/I2885cm−1) is shown as normal: ADC: SCC to be 0.81: 0.87: 1.31, respectively. The ratio of RR peaks of protein to lipid (I2934cm−1/I2851cm−1) is shown as normal: ADC: SCC to be 0.84: 0.94: 2.80, respectively. The result of these ratios reveals the changes of compositions of the macromolecules (e.g., lipids and proteins) during the evolution of the types of brain metastases of lung cancers of ADC and SCC. The lipid (2850 and 2885cm−1) content decreased in malignant ACC and SCC sharply (shown in Figs. 1(e) and 1(f)). This result is well consistent with the previous reports of Raman spectra of glioblastoma multiforme (GBM) and basal cell carcinoma ex vivo.10,18,19 The ratios serve as a key approach to predict the metastatic potential of brain cancer using VRR spectroscopy analysis.
VRR spectral fingerprint of amide II for ADC and SCC (4): Another important feature indicated by Figs. 1(d)–1(f) is the increase of the protein line in the amide II active bond at 1548–1554cm−1 in ADC and SCC in comparison with normal brain tissues. Intense resonance enhancement of 1555cm−1 band in SCC cancer and less increase in ADC cancer were observed. It reveals biomarker amide II and is important to differentiate ADC and SCC from normal brain tissues. Amide II is one of the nine amide bands of protein and was also resonance-enhanced in gliomas and meningeal cancers due to 532nm-excited amide II vibrational mode.10,17,18 Usually, the amide II band is weak or absent in the non-RR system.
Identification of human normal brain tissues and brain metastases of lung cancer by morphology (5): The images of Figs. 1(a), 1(g), 1(b), 1(h) and 1(c), 1(i) were taken from intracranial brain region of normal tissue and metastatic brain tumor of lung cancers of ADC and SCC, respectively. In the images of the normal brain tissue shown in Figs. 1(a) and 1(g), the main compositions such as proteins, lipids and lipoproteins, which make up neuronal and glial cells, are more ordered in layers and uniform in shape and size while these ordered features differ in cancer tissues. This can be observed in the images of ADC in Figs. 1(b) and 1(h) and SCC in Figs. 1(c) and 1(i), which display a periodic, but random, anti-symmetrical, and disordered structure. These features were also observed in prostate, breast and other types of tumors.55,56,57
The microscopic images with H&E staining used for the gold standard histopathology diagnosis are shown in Figs. 1(g)–1(i). It indicates that Figs. 1(h) and 1(i) for cancerous tissues lack normal tissue structure as shown in Fig. 1(g), and the brain cancer of ADC shown in Fig. 1(h) demonstrates points of gray–red tissues. Moreover, a few atypical cells can be seen among a large piece of necrotic tissue for SCC in Fig. 1(i), indicating that gray–red precipitate and metastatic-differentiated SCC is associated with necrosis in brain cancer tissues.
Identification of human normal brain tissue and brain metastases from lung cancers by SFSA (6): The analyses of the spatial frequency distribution by SFSA: In Fig. 2, the 2D spatial spectra were obtained using DFT in Eq. (2). By sampling with N=512, where N is a finite sampling extent, Figs. 2(a)–2(c) display the typical 2D amplitude spectra of normal, ADC and SCC of brain tissues, respectively. For visualization purpose, the amplitude spectra shown in Fig. 2 are exhibited as logarithms of original amplitudes28 and linearly mapped in the color range of [0, 255]. One-dimensional (1D) spatial frequency spectra (dot lines) through the peaks of the 2D amplitude spectra and their corresponding fitted Gaussian distributions (solid lines) are shown in Fig. 2 for (d) normal tissue, (e) ADC and (f) SCC.
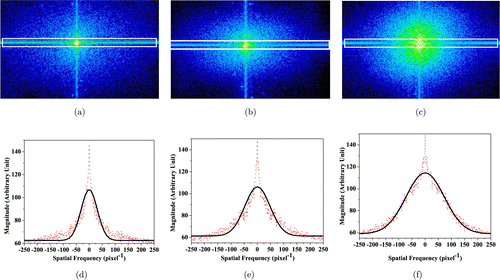
Fig. 2. (a)–(c) are 2D spatial frequency spectra obtained from their corresponding confocal microscope images shown in Figs. 1(a)–1(c), respectively; (b)–(d) are 1D spatial frequency spectra (dot line) along the horizontal direction through the peaks of the 2D spectra in (a)–(c), respectively, along with their corresponding fitted Gaussian distributions (solid line).
The dominant amplitude of the spatial frequency spectra is close to the origin (u=0, v=0) and decreases in all directions away from the center.31 These typical results of spatial frequency distribution clearly show strong signal at low spatial frequencies, but weak signal at high frequencies. These differences are also displayed in the 1D frequency spectra in Figs. 2(d)–2(f) that are generated from data shown in Figs. 2(a)–2(c). The results indicated a difference between the two classes of brain tissues including normal and cancerous tissues (with two subtypes of ADC and SCC). It is shown that higher frequency components are more contributive in brain cancerous tissues than in brain normal tissues. The SFSA results from Fig. 2 are summarized as follows: for the normal tissue, the lower frequency amplitudes mostly dominate over the mid-range and high-frequency range as shown in Fig. 2(a). However, the amplitude of mid-range and high-frequency range can be clearly perceived with the evolution from normal tissue in Fig. 2(a) to ADC in Fig. 2(b) and SCC in Fig. 2(c). These differences among the different types of tissues can be more distinctly seen from their 1D spatial frequency distributions along horizontal direction as shown in Figs. 2(d)–2(f), which are generated by integrating spatial frequency distributions over the vertical direction in the rectangle of solid line in Figs. 2(a)–2(c), respectively. It is shown that spatial frequency spectral profile of the SCC type of brain metastasis of lung cancer is wider than that of normal tissue.
In order to utilize the difference of the width of the spatial frequency spectra to differentiate the types of tissues, the width of the spatial frequency spectra needs to be quantified. The data shown in Figs. 2(d)–2(f) were fitted using Gaussian distribution with zero mean :
The differences in σ among different types of tissues were used to evaluate the tissue types. Figure 3(a) shows σ of normal (circle) and cancer (square) tissues, obtained by fitting the experimental data to the Gaussian function in Eq. (4). An increase in the spatial frequency range from normal to cancerous tissues is seen in Fig. 3(a). The expanding spectral range refers to more high-frequency components. This is called “broadening of the signal”.58 The spatial frequency spectra of cancerous tissues are wider when compared with normal tissue. This may provide a diagnostic criterion (σ) for distinguishing brain tumor tissues from normal tissue.
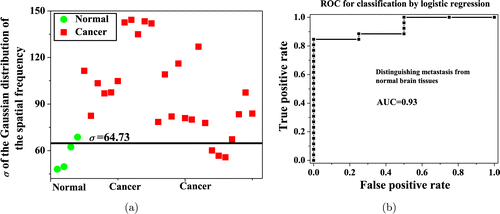
Fig. 3. (a) The σ of the spatial frequency spectra of four normal brain tissues and 26 cancerous brain tissues. The solid line is calculated using SVM to classify the two tissue types. (b) The ROC curve is used to evaluate the performance of the SVM model.
To evaluate the potential use of σ as a criterion, a support vector machine (SVM)59 is used to analyze the alterations of σ from normal to different types of cancerous tissues. In general, the SVM classifier is determined by a number of “data points” with the most effective discrimination, the support vectors (SVs), which are usually chosen from those at the boundary of the groups of data. In our study for separating normal and cancerous tissues, the SVs are chosen to be σ values between σCmin−k and σNmax+k, where k is a self-defined threshold value for optimum, and σNmin and σNmax are the minimal σ for cancerous tissues and maximal σ for normal tissues, respectively. All the analyses of SVM are performed in Matlab and were presented in detail in our previous study.60 This standard will be used to classify an unknown data set in the future study when a large number of samples is available.
Classification of metastatic brain tumors from normal brain tissues based on the SFSA–VRR data by SVM classifiers: The SFSA with SVM analysis on micrograph images is well consistent with the VRR with SVM analysis. The results of both methods were compared with gold standard reports.
In a diagnostic test, the outcome may be a positive predictive value (disease) or a negative predictive value (healthy), which can be either true or false. The sensitivity and specificity then can be calculated as
Diagnostic results of SVM classifier based on VRR18: The brain metastasis of lung cancer and normal brain tissue specimens were classified based on the VRR spectra using SVM.10,59,61 The SVM classifier used a Gaussian kernel and yielded diagnostic sensitivity, specificity and accuracy of 100%, 75.0% and 96.7%, respectively.
Diagnostic results of SVM classifier based on SFSA: To test the potential of the diagnosis method using σ, the criterion (threshold) for classifying the positive and negative groups in our study are determined using the SVM on all the tissue samples. It is shown that σ=64.73 denoted by the solid line accurately discriminates normal brain tissues from cancerous brain tissues in Fig. 3(a). Based on the two statistical measures, sensitivity and specificity, the accuracy of the categorization of the tissues was quantified. Subsequently, the receiver operating characteristic (ROC) curve is also generated as shown in Fig. 3(b) to evaluate the performance of the classification criterion based on the σ of the spatial frequency for distinguishing normal and cancerous brain tissues. The accuracy can be measured using the AUC (area under the ROC curve).
The sensitivity, specificity and the AUC values for SVM classification using the σ of the spatial frequency for brain tissues are summarized in Table 1.
| Evaluated components | Sensitivity | Specificity | Accuracy | AUC |
|---|---|---|---|---|
| Normal versus cancer | 88.5% | 75.0% | 86.7% | 0.93 |
The sensitivity, specificity, accuracy and AUC values in Table 1 demonstrate the excellent efficacy of SVM based on the spatial frequency as a promising diagnostic tool for brain cancer detection. Figure 4 shows the change in the criterion σ generated by SFSA method from normal to metastatic brain tumor tissues of ADC and SCC. An increase in σ of the spatial frequency is observed from normal to different types of cancerous tissues. This may provide an alternate diagnostic criterion for grading brain tumor tissues. Identifying the primary metastatic potential of cancer is critical in cancer detection and staging. This study is highly relevant to tumor grading by optical techniques and may serve as a key step to predict the primary metastatic potential of brain metastases. It helps in understanding the use of reflectance micrographic images and Raman techniques to identify potential metastatic cancer using intact tissue specimens.
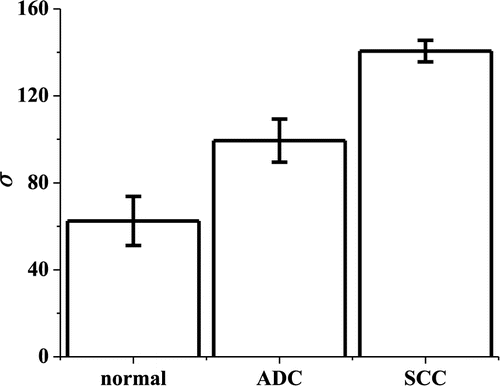
Fig. 4. The increase of σ of the spatial frequency spectra as a function of brain tissue type including normal brain tissue and brain metastases from lung cancers: ADC and SCC.
The SFSA–VRR results demonstrated consistency with the histopathology diagnosis (Figs. 1(g)–1(i)). Even though in this study, the classification was performed based on the SFSA of the micrographic images and VRR spectra separately, diagnostic information was observed using the synchronously carried-out SFSA and VRR techniques on the same sites. Therefore, this is a dual-modal technique for cancer diagnosis. A new classifier can be developed that combines the results of SFSA and VRR in future studies for more robust diagnosis.
4. Conclusion
VRR spectra demonstrate an array of biomarkers (e.g., carotenoids, tryptophan, amide II, lipids and proteins) functioning simultaneously in real time based on which it provides a label-free technique for differentiation of human brain normal tissues from the brain metastases of lung cancers.
An SFSA criterion parameter, σ, as a signature indicator is obtained by SVM based on the width of the Gaussian distribution fitted to the spatial frequency spectra for differentiation of human normal brain tissues from cancerous brain tissues. The SFSA and VRR-combined method can provide the results from simultaneous detections since micrograph images and VRR signals can be measured synchronously.18,62 More information can be found in Ref. 63. Based on this study, a new optical pathological method based on the dual-modalities of SFSA–VRR may be developed to improve the accuracy of brain biopsy in neuropathology diagnosis and to guide brain surgery.
Conflict of Interest
The authors have no associations with commercial companies that provided support for the work reported in the submitted manuscript or non-financial associations that may be relevant or seen as relevant to the submitted manuscript.
Acknowledgments
This research is supported by The Air Force Medical Center, China and in part of The Institute for Ultrafast Spectroscopy and Lasers (IUSL), the City College of the City University of New York. The authors would like to thank Mr. C. Y. Zhang, Mr. M. Z. Fan and Dr. X. H. Ni for their assistance in the experiments and suggestions concerning this paper.