Black phosphorus as a versatile nanoplatform: From unique properties to biomedical applications
Abstract
Black phosphorus (BP) or phosphorene, a new superstar among two-dimensional (2D) materials, has sparked huge scientific interest since its discovery in 2014. BP offers unique characteristics including high drug loading efficiency, excellent photodynamic and photothermal properties and good biocompatibility. These characteristics expand versatility of BP in nanomedicine. Although the outlook of BP seems promising, its practical biomedical applications are still at the very initial stage especially in comparison to other thoroughly investigated inorganic nanomaterials. This paper reviews BP structure and properties as well as its preparation approaches with the emphasis on techniques to improve BP stability and biocompatibility for their further usage in physiological environment. Meanwhile, recent progress made in various biomedical research fields from bioimaging to biosensing is discussed. Last, but not least, current challenges and prospects for BP in biomedicine are briefly examined, which will be useful to guide future developments of BP.
1. Introduction
Black phosphorus (BP) or phosphorene, a new superstar among two-dimensional (2D) materials, with its unique structure and properties, ignited huge scientific interest since its discovery in 2014.1,2,3 Each phosphorus atom in a single monolayer is covalently connected with its three neighboring atoms creating a bilayer-like puckered structures along the zigzag and armchair directions, respectively.4 Such structural anisotropy is responsible for exceptional mechanical,5 optical,6,7,8,9 electrical,10,11,12,13 and thermoelectric properties of BP,14 which distinguishes them from other 2D materials (such as molybdenum di-sulfide (MoS2), graphene, hexagonal boron nitride (h-BN) and tungsten di-selenide (WSe2)). Table 1 compares properties of BP with these similar materials.
| 2D materials | Composition and structure | Bandgap (eV)/Carrier mobility (cm2V−1s−1)/ON–OFF current ratio | Main preparation techniques | In vivo biodegradability | Biocompatibility | Main biomedical applications | References |
|---|---|---|---|---|---|---|---|
| BP | 100% P; puckered structure | 0.3–2.0/1000/103–105 | Liquid exfoliation, electrochemical method, chemical vapor deposition (CVD), plasma etching, mechanical exfoliation, and wet chemistry | Yes, producing nontoxic and biocompatible intermediate by-products (e.g., phosphites, phosphates and other PxOy) | Yes | Bioimaging, cancer therapy, drug delivery, and biosensing | 41,42 |
| Graphene | 100% C; hexagonal honeycomb | 0∕2×105/5.5–44 | Chemical, mechanical and liquid exfoliation, CVD, molecular beam epitaxy surface segregation | No, needs functionalization | Yes | Cancer therapy and biosensing | 43,44 |
| MoS2 | Molybdenum-sulfur transition metal dichalcogenides; hexagonal structure with Mo and S atoms positioned at alternating corners | 1.2–1.8/10–200/106–108 | Chemical, mechanical and liquid exfoliation; chemical and thermal vapor deposition | No, needs functionalization | Yes | Cancer therapy and biosensing | 45,46 |
| WSe2 | Selenium, tungsten transition metal dichalcogenides; hexagonal structure with W and Se atoms positioned at alternating corners | 1.2–1.7/140–500/104–106 | Mechanical exfoliation, CVD | No, needs functionalization | Yes | Cancer therapy, gene therapy and biosensing | 47 |
| h-BN | Boron nitride; hexagonal honeycombs | 5–6/-/- | Electron beam irradiation, liquid, chemical and mechanical exfoliation, CVD | — | Yes | Cancer therapy and bioimaging | 48,49 |
Significant amount of research was performed to investigate nano- and opto-electronic applications of BP such as photonics involving saturable absorption of BP.15,16,17,18 However, BP biomedical applications were developed less probably because of its insufficient stability in aqueous environment and/or in air.19,20 Only most recent studies started exploring feasibility of fabrication of novel BP nanostructures stable in air and water.1,21,22 Phosphorus is essential to our physiological processes as it is one of the major components of (deoxy)ribonucleic acids.23 Thus, phosphorus-based materials are expected to be biocompatible with a wider range of potential applications in biomedical fields.24,25,26 One of the examples is usage of excellent intrinsic electrochemical properties of BP for biosensing of nucleic acid,27 immunoglobulin G (IgG)28 and myoglobin (Mb).29 Another example is nano-BP usage for cancer therapy and drug delivery since it demonstrates high drug loading efficiency, excellent photodynamic and photothermal properties as well as good biocompatibility.30,31,32
Numerous inventions, innovations and discoveries in fundamental research and biomedical applications of BP were made judging by the growing relevant amount of scientific paper published since 2014. Additionally, practical applications of several platforms for cancer therapy and drug delivery based on nano-BP were also studied over the last couple of years.33,34 Biomedical applications of BP-based nanomaterials are still in their very first stages of development, and there is still myriad of technical challenges to be solved. However, if these challenges are unraveled, usage of BP-based nanostructures will undoubtedly yield many new opportunities for precise and efficient medical diagnosis and disease management. Solving these challenges will be only possible with undertaking extensive studies of BP properties, which will determine their specific biomedical applications, including wound healing,35,36 drug resistant bacteria,37 sonodynamic cancer therapy,38 and neurodegenerative diseases.39,40
This review starts with a discussion of BP structure and properties as well as its preparation approaches with the emphasis on techniques to improve BP stability and biocompatibility for their further usage in physiological environment. Recent progress made in various biomedical research fields is then discussed, including bioimaging, cancer treatment, drug delivery, neurodegenerative diseases, three-dimensional (3D) printing scaffolds and biosensing. In addition, current challenges and prospects for BP-based nanostructures in biomedicine are briefly examined.
2. Structure and Properties of BP
2.1. Structural characteristics
BP crystallizes in Cmca space group as a layered orthorhombic structure, which is very similar to the hexagonal layered structure of graphite with the exception of the “wrinkled” morphology (Fig. 1(A)).50 Uniqueness of BP structure is that it has a wrinkled morphology along the armchair direction and a two-layer pattern along the zigzag direction (Fig. 1(B)). Individual phosphorus atoms are covalently bonded with the other three phosphorus atoms. Several layers align parallel to each other by somewhat weak Van der Waals forces.51 Each phosphorus is connected to its three neighboring atoms forming bonds with two different lengths (R1 and R2) and with two different angles (θ1 and θ2) (Fig. 1(C)). Bonds with two out of three atoms are in the same plane. These phosphorus–phosphorus bonds (which are 2.224Å long) are marked as R1 in Fig. 1(C): they form a 96.3∘ hinge angle (θ2). The third bond connects to phosphorus atom located in the adjacent plane. This bond is marked as R2 in Fig. 1(C) and is 2.244Å long. Bonds R1 and R2 form dihedral angle (θ1) equal to 102.095∘.52 Length of these bonds and values of these angles vary depending on number of layers.
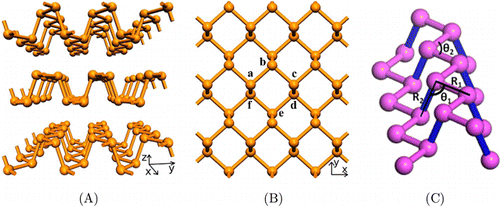
Fig. 1. Ball and stick schematics of BP crystal structure: (A) side and (B) top views (adapted from Ref. 50 with permission). Six phosphorus atoms form a space-distorted honey-comb hexagonal shape. In (B), the atoms located in the upper and lower planes of this honey-comb cell are indicated as a, b, c and as d, e and f, respectively. Armchair direction is along the x-axis, and zigzag direction is along the y-axis. (C) Spatial schematic of relative location of R1 and R2 bonds and θ1 and θ2 angles (adapted from Ref. 52 with permission).
2.2. Properties of BP
2.2.1. Mechanical characteristics
The monolayer BP demonstrates 27% and 30% tensile strain in the zigzag and in the armchair directions, respectively (Fig. 2(A)-(a)). The critical strains of multi-layer BP are 24% in the zigzag direction and 32% in the armchair direction (Fig. 2(A)-(b)).5 It has flexibility superior to that of graphene as well as smaller Young’s modulus than graphene: 0.166TPa and 0.044TPa in zigzag and in armchair directions, respectively. In-plane Young’s moduli in the directions perpendicular (which corresponds to the armchair direction) and parallel (which corresponds to the zigzag direction) to the pucker are equal to 41.3GPa and 106.4GPa, respectively.53 Puckered structure of BP is the reason for such anisotropy: during mechanical compression and tension, this puckered structure undergoes multiple folding/unfolding cycles along the armchair direction.

Fig. 2. (A) The strain–stress relation for (a) monolayer and (b) two-layer BP structures. Adapted from Ref. 5 with permission. (B) Electronic structures of few-layer BP. (a) and (b) Bandstructures of monolayer and biolayer BP calculated with the HSE06 functional (red solid lines) and the mBJ potential (blue dashed lines), respectively. Adapted from Ref. 59 with permission. (C) Plot of the thermal conductance in the zigzag and armchair direction as a function of temperature at different strain values (0%, 2% and 4%) for uniaxial strain applied in the (a) zigzag and (b) armchair direction. Adapted from Ref. 60 with permission. (D) Optical absorption spectra. (a) and (b) Optical absorption spectra of few-layer BP for light incident in the z direction and polarized along the x and y directions, respectively. Adapted from Ref. 59 with permission.
It was further reported that functionalization can modify BP Young’s modulus.54 For example, sodiation can increase or reduce Young’s modulus values depending on how many Na atoms BP monolayer adsorbs. However, functionalization cannot eliminate the anisotropy,54 which is a disadvantage since it makes BP mechanically weak in general and especially in the armchair direction.55
However, Li et al.56 found a solution to decrease this anisotropy to some extent. They produced angle-ply double-layer BP material by stacking two individual monolayers with different orientation angles. As a result, an anisotropy was decreased. In fact, degree of anisotropy reduction correlated to the value of the stacking angle δ between the two layers: at higher angles, less anisotropy was observed, and at δ=90∘ BP became isotropic. The resulting BP structure was more ductile in armchair direction and exhibited an abnormally negative value of Poisson’s ratio for in- and out-of-plane directions parallel to the pucker.57 Such Poisson’s ratio also confirms BP anisotropic behavior.58
2.2.2. Electronic characteristics
BP has a direct bandgap, which is dependent on the number of layers in any particular BP-based material: it ranges from 0.3eV (for bulk BP) to 2.0eV (for a monolayer BP). Thus, BP bandgap decreases as the number of layers increases (Fig. 2(B)).61
External electric field also affects BP bandgap, and this effect also depends on how many layers BP-based structures have. For example, external electric field does not change bandgap of a BP monolayer. However, bandgap of BP with several layers changes significantly. In fact, BP with just several layers can be converted into a topological insulator and, finally, into a metal upon its exposure to the external electric field.41 Liu et al.62 demonstrated how a typical insulating material can be converted into a topological insulator. Their results provide basic principles applicable to variety of materials for development of multifunctional topological field-effect transistors.
Chemical modifications can also change bandgap of BP. For example, Jing et al.63 demonstrated that by modifying surface of mono- and several-layer BP-based materials with tetracyanoquinodimethane (TCNQ), tetracyanoethylene (TCNE) and tetrathiafulvalene (TTF), their bandgap values can be decreased substantially. Thus, surface modification of BP can enhance its general and direction-specific light absorption properties.
Electron transport behavior of BP also demonstrates a substantial anisotropy associated with unique ridge structure of BP. Single layer of BP also exhibits on/off ratio up to 10,000 at room temperature as well as high mobility of ∼10,000 cm2/Vs along the armchair structural direction.59 Yet, most properties of BP as well as its structure are diminished quickly upon BP exposure to atmospheric conditions and/or high-density charge traps. Thus, its mobility becomes limited as well.
However, it was demonstrated that stabilization of sandwiched BP heterostructure by encapsulating it with thin boron nitride (BN) layer can result in high field-effect mobility of BP (equal to ∼1350 cm2V−1s−1 at room temperature) as well as on–off ratios over 100,000 even when this BP-based material is operated at ambient conditions.64 Other studies showed that nanoholes can modify electronic structure of BP nanoribbons as well as their responses to external electrical fields and strains, which makes BP nanoribbons promising materials for nano- and opto-electronic applications.65 in which high and long-term stability of the material is required.
2.2.3. Thermal characteristics
Thermal properties of BP are anisotropic and depend not only on the direction but also on size and strain of the BP-based materials. Figure 2(C) shows the thermal conductance in the zigzag and armchair direction as a function of temperature (T = 10 K to 1000 K) for strain applied in the zigzag and armchair directions.60 BP thermal conductivity in the zigzag direction is much larger (by up to 40%) than in the armchair direction.60 Anisotropy ratio of BP equal to ∼3 is probably the largest for any known material: predicted values in zigzag and armchair directions at 300 K were estimated to be ∼110 W/m-K and 36W/m-K, respectively.66 Both of these values are larger than those for silicene, close to those of MoS2 and almost two times lower than those of graphene.67 Thermal conductivity in the zigzag direction depends on the size (it increases for samples with long particles), while in the armchair direction it does not depend on the particle size.68 However, upon tensile strain, thermal conductivity significantly decreases in both directions.69 For example, at ∼60% uniaxial tensile strain in the armchair direction, thermal conductivity of phosphorene decreases ∼4.7 and ∼2.2 times along the armchair and zigzag directions, respectively.66 According to a recently-reported study, thermal conductivity of BP can be also influenced by an external magnetic field.70
2.2.4. Optical characteristics
Materials based on BP also demonstrate anisotropic optical responses: BP absorbs polarized light in the armchair direction. However, it transmits polarized light in the zigzag direction.71 Because of absorption in the infrared (IR) and in the visible light spectra, BP can potentially be applied as active material in optical linear polarizers.72,73,74 Absorption spectra of BP also demonstrated highly anisotropic nature (Fig. 2(D)),59 thus, ∼15nm BP thin films can be ideal candidates for variety of near- and mid-IR optoelectronic devices (e.g., modulators, photodetectors, etc.).18,75,76,77
Optical properties of monolayer BP also strongly depend on applied strain.78 Thus, biaxial strain can tune optical bandgap of BP consisting of just one atomic layer from 0.38eV (at −8% strain) to 2.07eV (at 5.5% strain). Tensile strain can also improve electron transport along the zigzag direction. When strain changed from compressive to tensile, optical bandgap can change by ±1.5eV. Thus, strain engineering can tune BP optical response.
3. Preparation Techniques
BP can be synthesized using either bottom-up or top-down techniques.79 In general, during top-down approaches, bulk BP is exfoliated (by mechanical or liquid exfoliation) into mono- or several-layered sheets. Top-down methods are typically plasma-assisted one including plasma etching, electrochemical, mechanical and liquid exfoliations. Bottom-up methods (e.g., CVD) generally are fabrication of nanomaterials from specific precursors. The following section discusses drawbacks and advantages of these approaches in terms of different applications of their final products.
3.1. Liquid exfoliation
Techniques involving liquid exfoliation produce substantial quantities of 2D materials.80,81,82,83 These methods are inexpensive, easy to implement and to scale, and, what’s most importantly, allow exfoliated nanosheets to be post-processed using existing industrial methods.84,85,86 Liquid exfoliation can be categorized into three different groups: (1) ion intercalation and (2) exchange as well as (3) ultrasonication-assisted methods. Typically, layered materials contain ions in the layers to maintain and to balance surface charges of these layers (shown as yellow spheres in Fig. 3). Crystal structure will become weaker when placed in a liquid because of compromised interlayer, which might lead to swelling of the BP-based materials. Agitation (by heating, by shear or by ultrasonication) will completely separate these swollen layers and result in exfoliated single- or several-layer particles (Fig. 3(a)). These interlayer ions (shown as red ones in Fig. 3(b)) can also be exchanged with larger ions (shown as yellow spheres in Fig. 3(b)) and further exfoliated as described above using various solvents. The resulting nanosheets can be well-stabilized against aggregation when exfoliation is performed in solvents with compatible surface energy. If precautions were not taken or if exchange-ion choice was inappropriate or the solvent surface energy is not compatible with the system, secondary aggregation and/or sedimentation can occur (Fig. 3(c)).87 One of the examples of liquid exfoliation of BP is 4h ultrasonication of suspension of 5mg/mL bulk BP (with density 0.164mol/dm3) in N-methyl-2-pyrrolidone placed in water bath below 30∘C.88 The resulting precipitates were separated from the mother solution, cleaned and fractioned (to remove large particles) by centrifugation. The final product was yellow–brown liquid.

Fig. 3. Liquid exfoliation method to obtain nanosheets. (a) Ion intercalation (b) Ion exchange (c) Exfoliation by ultrasonication. Adapted from Ref. 87 with permission.
Liquid exfoliation is also very successful to obtain ultrathin BP nanosheets. A very novel method involving liquid exfoliation with phytic acid is capable to produce ultrathin and uniform BP nanosheets up to several tens of micrometers wide and/or long with very exciting future applications in photocatalysis and photovoltaics.89 Another study used liquid exfoliation technique to fabricate highly crystalline few-layer BP (FLBP) thin films with a triangular crystalline structure directly on SiO2/Si (001) and amorphous carbon.90 Such composites are important for novel sensor-related applications.
Liquid exfoliation methods are often combined with microwave- and shear-assisted techniques to obtain BP monolayers. Thus, high-quality with just a few layers of BP flakes (with average sizes below 10nm) with excellent stability were prepared in common organic solvents using only short exposure to microwave radiation.91
Despite the fact that bulk BP could be relatively easy exfoliated, reactivity of the resulting BP flakes at even mild (e.g., ambient) conditions limits their widespread direct applications. However, some prevention measures can be still undertaken. Thus, in one work, self-assembled 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) layer was deposited on the surface of FLBP during liquid exfoliation to prevent water and/or oxygen from reacting with FLBP.92 This approach significantly improved stability of the passivated FLBP. Sulfur-doped BP nanosheets, prepared by high-pressure method with liquid exfoliation as a final synthesis step, also demonstrated excellent stability in ambient conditions.93 These structures were used as stable and efficient catalysts for oxygen evolution reactions.
3.2. Electrochemical exfoliation
Electrochemical exfoliation is also often used to prepare nano-structured BP.94 One of the specific methods is BP synthesis by thermal treatment of red phosphorous (Fig. 4(a), left image and left photograph). This two-electrode setup consisted of BP flake as an anode (attached to the system using Cu tape), Pt cathode and H2SO4 as electrolyte (Fig. 4(b)). Both electrodes were parallel to each other and 2cm apart. First, positive 1V DC voltage was applied for 2min to ensure wetting of the BP crystal. Then, after the voltage was increased to +3V, formation of fine material became noticeable around BP crystal, and the solution color changed slowly to yellow–orange (Fig. 4(c)). This process proceeded without any further voltage changes. Such reaction flow is very beneficial to the overall process since higher potentials can cause oxidation of freshly-formed BP sheets. After 2h, the solution became dark-orange and a lot of particulates could be clearly observed at the bottom of the cell (Fig. 4(d)). This precipitate was washed with deionized water, separated from the mother solution and dried using vacuum filtration. The resulting exfoliated particles were then dispersed in dimethylformamide by ultra-sonication. After 1h of sonication, clear greenish–gray dispersion was obtained (photograph on the right in Fig. 4(a)).95

Fig. 4. Electrochemical exfoliation to obtain nanolayers. Layered crystal structure of black phosphorous is exfoliated in acidic aqueous solution by the application of a DC voltage. (a) Pictures of the starting BP crystals (left) and the exfoliated material dispersion in DMF (right) are also shown. Snapshot of the electrochemical setup with BP flake anode and Pt foil cathode separated in acidic solution (0.5M H2SO4) by a fixed distance of 2cm at (b) no potential applied, (c) after 20min applying a voltage of +3V and (d) after 2h process. Adapted from Ref. 95 with permission.
Another study developed quick and easily-scalable electrochemical method for BP nanosheet fabrication. In this method, BP served as a cathode. The resulting exfoliated BP nanosheets contained less oxygen than initial bulk BP, demonstrating that oxidized or structurally and chemically compromised BP nanosheets can be restored to some extent. Defect-, degradation- and oxygen-free BP is, undoubtedly, more efficient for (opto) electronic and other applications.96
3.3. Plasma etching
Plasma etching is a relatively new technique to fabricate air-stable BP films with outstanding properties and with a desired number of layers (Fig. 5).97 Typical procedure of a plasma etching fabrication method typically follows a simple route: first, a monolayered exfoliated BP flake is placed onto a SiO2/Si substrate and treated with oxygen plasma (Fig. 5(a)), during which the top layers of BP oxidizes to PxOy, forming a thin protective layer. Continuing plasma etching increases thickness of the surface PxOy, since plasma penetrates deeper into the underlying BP. At the same time, due to continuous collisions with oxygen plasma, top PxOy layer detaches from the rest of the material. Thus, during plasma treatment, a dynamic equilibrium is achieved between BP oxidation and rate of PxOy removal. Eventually, both thickness of the PxOy layer and etching rate become constant (Fig. 5(b)), which help to precisely fabricate any number of BP layers (Fig. 5(c)), including a monolayer BP. Degradation of the layers underneath PxOy is always inhibited because PxOy protects them.

Fig. 5. Plasma etching to produce BP samples stable at ambient conditions. (a) A thick phosphorene flake is firstly exfoliated onto a SiO2/Si substrate and the sample is then treated with O2 plasma etching (yellow balls). (b) During the O2 plasma pre-treatment process, the top layers of the phosphorene flake are oxidized to become PxOy, which then serves as a protective layer for the remaining phosphorene sample underneath. With further O2 plasma etching, oxygen plasma can penetrate the PxOy layer by diffusion and oxidize the underlying phosphorene, and this thins down the phosphorene layer and also increases the thickness of the PxOy. Meanwhile, the O2 plasma physically sputters away the PxOy layer from the top because of the collisions by oxygen plasma. After the plasma pre-treatment, a dynamic equilibrium is reached between oxidation of the phosphorene and physical removal of the PxOy layer, such that the PxOy layer approaches a constant thickness and the etching rate also becomes constant. (c) Because of the constant etching rate, any designated number of layers of phosphorene down to a monolayer can then be precisely fabricated and the degradation of the remaining layers is inhibited because of the protective nature of the PxOy. (d) To further improve the lifetime of the phosphorene so-produced, the sample was also coated with an Al2O3 protective layer by atomic layer deposition (ALD). In this case the PxOy layer prevents the underlying phosphorene from reacting with the precursor gases used in the ALD process, which is especially important for samples less than a few layers thick. Adapted from Ref. 97 with permission.
A second typical step following plasma etching is to coat BP with another thin layer, for example, with Al2O3 by ALD. PxOy again acts as a protective layer preventing underlying BP layers from reacting with the ALD precursor, which is especially critical for samples with only few layers thick (Fig. 5d). Plasma treatment not only controls BP film thickness, but also removes oxidized or degraded parts of the BP layer itself, which on a big scheme of things, helps to obtain nano-BP-based devices with enhanced field-effect transistor-like performance.98 Another study concluded that oxygen plasma treatment is more suitable for BP nanocrystals fabrication with desired thicknesses.99 BP nanocrystals obtained using plasma etching are simultaneously photo-luminescing (at new wavelengths) and electrically conductive.
3.4. Mechanical exfoliation
One of the earliest and most frequently used methods of 2D materials fabrication (including BP nanosheets) with high purity is indeed mechanical exfoliation. This technique can deliver nanosheets with reasonably large areas (Fig. 6).100 The drawbacks of mechanical exfoliation, which limit its wider and larger-scale applications, include low yield and inability to control size, thickness and shape of the resulting BP nanosheets with enough accuracy and reproducibility. An additional disadvantage of this approach is chemical cross-contamination from the bonding agents of the tapes used to separate the layers.

Fig. 6. Mechanical exfoliation of bulk BP to obtain ultrathin BP nanosheets. Adapted from Ref. 100 with permission.
Several investigations focusing on optimization of the nanosheet shapes and on improvement of flake deposition were recently reported.101 One study developed a technique, which included mechanical exfoliation step followed by argon-plasma treatment with the goal to produce a stabilized monolayer BP nanosheet.102 In another study, bulk BP was sliced multiple times with blue Nitto tape, which was then pressed against polydimethylsiloxane (PDMS) substrate to transfer BP sheets from the Nitto tape (This is a rapid exfoliation step). PDMS substrate with freshly deposited BP nanolayers was then brought in contact with the new substrate for another more gentle transfer (This step is called a “slow” exfoliation step).103 Such multi-step exfoliation technique provides higher yield of flakes just few monolayers thick in comparison with transitional mechanical exfoliation.
Another study baked 1μm thick polyvinyl alcohol (PVA) at 70∘C for 5min, after which it was coated with polymethylmethacrylate (PMMA) followed by another 70∘C/5min baking step.104 Preliminary exfoliated BP flakes were then moved to the PMMA/PVA composite. This three-layer assembly was finally transferred to a free-standing Si3N4 substrate, then immersed in acetone for 12h to detach BP nanosheets from PMMA/PVA and finally dried with N2. The resulting S3N4/nano-BP composites were characterized by transmission electron microscopy (TEM) and Raman spectroscopy.
3.5. Chemical vapor deposition (CVD)
CVD is a typical bottom-up technique to obtain 2D nanomaterials on any substrate. Generally, a specific precursor and high temperatures are needed to implement this method. This approach produces extremely thin nanomaterials with high surface area and excellent crystallinity. This method also allows to tune thickness and size of the resulting 2D materials.
One example of a CVD procedures reported in the literature involves 500mg of red phosphorus, 10mg of SnI4 and 364mg of AuSn alloy, which were placed in quartz crucible and evacuated. The crucible was then heated very slowly to 873K over 10h-period, maintained at this temperature for one day and then cooled to 773K (Fig. 7).100 Sizes of the resulting BP nanocrystalline flakes, accumulated at the end of the sealed quartz crucible, were >1nm.101 Using CVD, Sinha et al.105 developed quick and versatile technique to passivate thin BP flakes by growing large sheets of high-quality h-BN monolayer directly on BP.

Fig. 7. Schematic of a bottom-up CVD approach showing precursor molecules. Adapted from Ref. 100 with permission.
To summarize fabrication techniques of BP, one can conclude that liquid exfoliation demonstrates several significant advantages such as low cost, simplicity and scalability. Liquid exfoliation can also produce thin films as well as hybrid composites. However, it uses organic solvents, most of which are hard to remove after the synthesis especially without traces. Thus, usage of BP obtained using liquid exfoliation might be problematic for the biomedical applications. Main advantages of electrochemical synthesis methods involve rapidness of the whole procedure and high yield of a product with excellent crystallinity. Plasma etching approach results in monolayer- or FLBP with high quality, however, it is impractical for industrial scale production. Fabrication methods involving mechanical exfoliation can generate nanosheets with reasonable large surface-to-volume ratios. Yet, it has low yield and does not provide enough control for BP nanosheet size, morphology and thickness. Thus, mechanical exfoliation is also problematic for mass production. In general, all bottom-up techniques are very complex and difficult to realize for industrial scale particularly because of their inability to obtain BP nanosheets with large areas on a large-scale with controllable layer thickness.
4. Instability Issue and Approaches to Overcome It
4.1. Mechanism of BP instability
Even though BP-based materials have numerous unique properties, instability of BP upon exposure to oxygen, light, water and/or even slightly elevated temperatures impedes its wide-spread uses.23,31,106
Oxygen affects BP surface in a most aggressive way especially comparing to all other external factors.107 Upon interaction with BP surface, O2 molecules distribute easily with perpendicular conformation along the top BP layer, which is very beneficial for a quick BP conversion to its oxidized form.108 This top layer is often called oxidized phosphorene or phosphorene oxide. Interaction with water also significantly contributes to BP instability. BP surfaces with ad- or chemisorbed oxygen are hydrophilic, thus, they demonstrate high affinity towards interaction with water, which induces fast BP degradation. Even nonoxidized BP demonstrates high water affinity because of strong dipole–dipole interaction between phosphorus and H2O. In fact, when BP was placed in humid environment, 200% volume increase was observed because of H2O adsorption.109 However, BP shows much better stability when in contact with de-aerated water.110 Light and temperature sensitivity of BP was confirmed by Favron et al., which showed even faster H2O- and O2-induced BP degradation upon BP exposure to light.111 These factors need to be taken into account during synthesis or fabrication of BP-composites for drug delivery.
4.2. BP stabilization approaches
Current methods to stabilize BP is simply to passivate its top layer by masking it with inert material such as graphene, Al2O3, h-BN, etc. For example, hybrid aerogels consisting of BP nanoflakes covered with graphene oxide (GO) demonstrated excellent stability and negligible degradation in ambient environment.20 These passivation layers can be as thin as one monolayer, which was demonstrated by a recent study involving usage of h-BN and graphene to passivate ultrathin BP to improve its stability.112 BP encapsulation with Al2O3 also provides very stable BP.113
Chemical functionalization, modification or coordination by various agents (such as aryl diazonium,114 titanium sulfonate lignands,115 polyethylene glycol (PEG)-amine,116 anions,19 etc.) is another strategy to stabilize BP. Doping (e.g., with metals, such as scandium117 or nonmetallic elements, such as sulfur118) is also reported as an efficient method to avoid or slow down BP degradation.
Despite variety of methods of stabilization mentioned above, BP degradation in physiological environment still remains an issue for its advances in biomedical applications.119 Therefore, additional methods to minimize or even to eliminate completely BP degradation are needed. One of such methods, namely, surface modification of BP with a polydopamine, significantly enhanced stability of bare BP nanosheets.120 BP nanosheets can also be covered with polydopamine to be used for drug delivery experiments.30 Some other novel stabilization and/or passivation methods include usage of coupling of BP quantum dots (BPQDs) with PEG, which resulted in significantly enhanced physiological stability.121 In another study, BPQDs, mixed with polyvinylpyrrolidone, demonstrated improved stability in N-methyl–2-pyrrolidinone.122
5. Biocompatibility
In general, toxicity of BP depends on its particle sizes, concentration in any given environment and/or system and interaction with specific cells.24,123 Systematic study to assess cytotoxicity of BP was conducted using mammalian NIH 3T3 cell line as well as HCoEpiC and 293T human cell lines. The highest cytotoxicity was detected for the thickest BP nanosheets with the largest lateral size.124 Sensitivity of these cells towards BP was in the following order: 293T > NIH 3T3 > HCoEpiC. Mechanisms responsible for induced in vitro cytotoxicity of BP might include (1) formation of intracellular reactive oxygen species (ROS) and (2) complete or partial disintegration of cell membranes upon their interaction with BP.125,126 Thus, for biomedical applications, size and concentration of BP are also needed to be optimized.
Cytotoxicity evaluations involving C2C12 skeletal myoblasts demonstrated dose-dependent cytotoxicity of present-in-the cell BP nanodots: they were cyto-compatible at concentrations below 4μg/mL.127 Another study also demonstrated BP content-dependent cytotoxicity, which was observed by exposing (for 24h) epithelial cells of human lung carcinoma (A549 cells) to various contents of BP. Upon exposure to 50μg/mL of BP, 48% and 34% of the cells died when water-soluble tetrazolium salt (WST-8) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were used, respectively.128 Sun et al.121 studied how long cells of hepatic stellate (HSCs), glioma (C6), human embryo kidney (293T), and breast cancer (MCF-7) will survive upon their incubation with 20–200ppm of PEG-enhanced BPQDs. After 48h, all these cells were still viable even when they were incubated with 200 ppm of PEG-modified BPQDs. Thus, specifically modified BP with certain particle sizes can indeed demonstrate low cytotoxicity and be biocompatible and suitable to be used in biomedicine.
BP easily biodegrades creating nontoxic and biocompatible by-, intermediate- or final decomposition products like phosphites, phosphates and various PxOy species.129 Thus, BP-based materials are very appropriate choices to be used in vivo. Bio-safety of drug delivery systems based on BP-platforms was demonstrated experimentally.130,131 A very thorough and systematic study was performed to access in vivo toxicology of a composite consisting of BPQDs and poly (lactic-co-glycolic acid) (PLGA) nanospheres (BPQDs/PLGA NSs).132 Blood of several mice was tested for standard hematology markers (red and white blood cells, hemoglobin, mean corpuscular volume as well as hematocrits and platelets) after BPQDs/PLGA NSs was intravenously injected into them. The results showed no infection and inflammation in these mice (Fig. 8(a)). Biochemical analysis of blood of the same mice (which included analysis for aspartate and alanine transaminases, total protein and bilirubin, blood urea nitrogen, albumin and creatinine as well as globulin) demonstrated that mouse treatment with BPQDs/PLGA NSs did not have any noticeable effect on their metabolism (Fig. 8(b)). Additionally, no visible damages were detected for major organs during the duration of the treatment, which implies absence of lesions or histological abnormalities in mice treated with BPQDs/PLGA NSs (Fig. 8(c)). Thus, good compatibility of BPQDs/PLGA NSs with mouse metabolism was experimentally confirmed.

Fig. 8. Results of in vivo toxicity studies. All blood and organ samples were taken 1, 7 and 28 days after the injection. Heights of the bars reflect average values; error bars correspond to the standard deviations. (a) Hematological data of blood of the mice intravenously injected with the BPQDs/PLGA NSs. The following abbreviations were used for the y-axis titles: WBC for white blood cells, RBC for red blood cells, HGB for hemoglobin, MCV for mean corpuscular volume, MCH for mean corpuscular hemoglobin, MCHC for mean corpuscular hemoglobin concentration, PLT for platelets, HCT for hematocrits. (b) Biochemical analysis of blood taken from NS-treated mice. The following abbreviations were used for the y-axis titles: ALT for alanine aminotransferase, AST for aspartate aminotransferase, TP for total protein, GLB of total globulin, TBIL for total bilirubin, ALB for albumin, BUN for blood urea nitrogen and CREA for creatinine. (c) Eosin and hematoxylin stained images from kidneys, spleens, livers, hearts, and lungs of NS-treated mice. Scale bar corresponds to 100μm. All graphics was adapted from Ref. 132 with permission.
To evaluate potential in vivo side effects, nanosystem consisting of PLGA and ultra-small BPQDs (PLGA-SS-D@BPQDs) was developed and tested on mice.130 After the treatment, which included twice-a-day injections of 20μg/kg of PLGA-SS-D@BPQDs for 21 days, main organs of mice looked healthy without abnormalities, inflammations or impairments. Another BP-based composite with good biocompatibility and biodegradable was produced by Xie and coworkers.133 Their composite consisted of piperazine-based polyurethane (PU) incorporated into BP sheets. The resulting composite was near-infrared (NIR)-photo-responsive memory polymer (SMP), in which PU acted as a thermo-responsive part of the SMP, and BP sheets player acted as NIR photothermal nanofillers. In vivo toxicology experiments showed excellent biocompatibility of this composite: its constituent parts, PU and BP, slowly decomposed into CO2 and H2O, respectively. BP nanosheets with liganded titanium sulfonate (TiL4@BPs), fabricated in another study, also demonstrated excellent biocompatibility. In addition, these composites escaped from uptake by macrophages, which always effectively reduces proinflammation and cytotoxicity. Moreover, no undesirable immune responses were observed.134 Thus, in vivo biodegradability and overall biocompatibility, confirmed by absence of adverse physiological effects upon mouse treatment by BP-based composites and materials, was demonstrated by several studies. Hence, BP-based systems have very strong potential as drug delivery systems.
6. BP Applications in Bioimaging
Because of unique electronic as well as optical properties, BP can be used for bioimaging. Principles of several key biomedical applications (such as photoacoustic (PA) and thermal imaging) are built on strong interactions between electromagnetic waves and BP crystal structure. These interactions exist in a wide energy spectrum: ultraviolet (UV) to visible to NIR region. Interaction over such a wide spectrum allows BP to have large extinction coefficient and large photo-redox potential. Methods like fluorescent imaging demonstrate BP layer-dependent fluorescence: spectrum intensity increases exponentially as nano-BP-based system becomes thinner.135 This section of our review describes PA, thermal and fluorescent imaging techniques involving BP.
6.1. Photoacoustic (PA) imaging
As a new bioimaging treatment, PA imaging combines advantages and overcomes disadvantages of other imaging analysis and diagnostic techniques: speckle artifacts occurring during ultrasound imaging and limited penetration depth occurring during optical imaging.136 PA method provides high spatial-resolution images of tissues with up to 5–6cm optical contrast depth as well as characteristic background-free detection137 Combination of PA imaging with BP provided even more benefits especially for in vivo diagnostics of tumors. For example, PEG-modified BP nanoparticles were used for cancer therapy.138 PA signal was significantly improved as concentrations of BP nanoparticles were increased from 0μg/mL to 250μg/mL (Fig. 9(A)-(a)). This increase demonstrated a very strong correlation with the BP nanoparticle concentration. Such great results obtained in vitro indicated great potential for these nanoparticles for in vivo PA imaging. In fact, injection of PEG-modified BP nanoparticles into mice with tumors in different organs enhanced imaging contrasts in their livers and kidneys as well as in tumor regions (Fig. 9(A)-(b)). The contrast became better as circulation time of PEG-modified BP particles in the mouse bodies increased, which implies gradual nanoparticles aggregation in these organs. Twenty-four hours after the injection, intensity around tumors was higher than in the kidney and liver regions, indicating higher BP nanoparticle content in the tumors. Thus, PEG-modified BP nanoparticles demonstrated excellent retention time in tumors and were easily removed from the circulation by mouse kidneys and livers.

Fig. 9. (A) PEG-modified nano-BP as a contrast agent for (a) in vitro PA imaging of cells with different concentrations of nano-BP and (b) in vivo PA imaging of kidneys and livers of mice as well as their tumors before and 15min, 30min, 2, 4, 6 and 24h and after the intravenous injection of PEG-modified BP nanoparticles. Adapted from Ref. 138 with permission. (B) Data from in vivo PA signals obtained using TiL4@BPQDs. (a) Time-dependent PA images of MCF-7 cells in the xenografted tumor of the Balb/c nude mice after they were intravenously injected with TiL4@BPQDs. Intensity scale is on the right-hand side. (b) Intensity of ROI signals before and 30 min, 1, 2, 4, 6, 24 and 48h after the injection. (c) Typical 3D PA images of tumors before and 4h and 48h after the injection with TiL4@BPQDs. Adapted from Ref. 139 with permission.
Composites already mentioned in this review consisting of titanium ligands (TiL4) coordinated with BPQDs (TiL4@BPQDs) also demonstrated excellent in vivo and in vitro PA ability to detect MCF-7 cancer cells and Balb/C nude mice.139 Intensities of PA signals from tumors after TiL4@ BPQDs were injected into mice were higher in comparison with PA signals recorded prior to the injection (Fig. 9(B)-(a)). The highest intensity was detected 4h after the injection (equal to 120.80±5.40, Fig. 9(B)-(b), after which PA intensity decreased and returned to the initial level after 48h. Before the injection and 48h after the injection, PA intensities in the tumors were 21.49±3.16 and 31.58±2.42, respectively. 3D imaging also confirmed PA signal increase in the tumors after TiL4@BPQDs injections (Fig. 9(B)-(c)). It was reported that high levels of BPQDs could be also accumulated in tumors because of the retention and permeability effects. Thus, BP demonstrated high promise for clinical applications since it provided enhanced spatial resolution needed for tumor detection as well as improved sensitivity for PA diagnostic techniques.140
Even through tumors themselves emit PA signals, their intensities are somewhat low especially during early cancer stages. Thus, enhancement of traditional PA imaging with contrast agents is becoming more and more popular. Such agents can be noble metal nanoparticles and organic and nonorganic dyes and composites, which strongly absorb in the NIR region and enhance PA response.137 Some PA enhancing agents are BP-based. One example is application of a network of a complexes (containing tannic acid (TA) and Mn2+-chelating agents) directly onto BP nanosheets forming BPNS@TA-Mn composites with improved contrast and excellent PA imaging abilities.141 Thus, one can confidently conclude that BP-based materials are very promising candidates to be used as theranostic agents.
6.2. Thermal imaging
Thermal imaging is sensitive and accurate technique, which measured the temperature difference of different body parts. Equipment associated with thermal imaging collects spatially-distributed IR radiation, processes it and generates an image showing location, features and progression of lesions. Thermal imaging plays a very important role in biomedical applications since it is noninvasive, which can perform whole-body diagnostics, sensitive and responsive to only temperature changes. Excellent optical absorbance characteristics of BP allowed it to be an excellent supplement to in vivo thermal imaging techniques.142,143
Our research group reported smart NIR-controlled drug-release BP@Hydrogel system, which consists of a low melting-temperature agarose and PEG-modified BP nanosheets. We tested this system for cancer treatment.116In vivo photothermal effects of BP@Hydrogel were investigated by injecting BP@Hydrogel and free doxorubicin (DOX) into the tumors (Fig. 10(A)-(a)). NIR radiation and temperature difference (ΔT) were detected using thermal camera. ΔT of the tumor with DOX was only ∼5∘C, while tumor containing BP@Hydrogel showed over 13∘C temperature difference, which implies that tumor containing BP@Hydrogel heated more upon NIR irradiation (Figs. 10(A)-(a) and 10(A)-(b)).
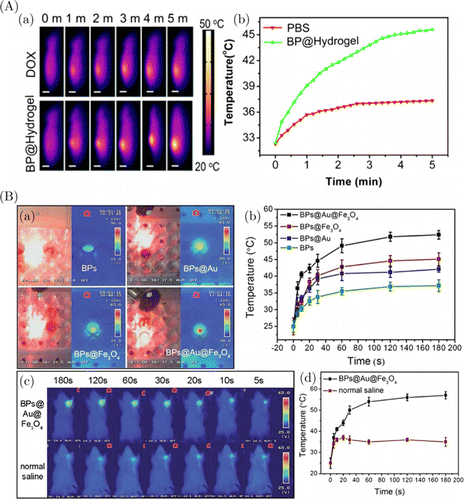
Fig. 10. (A) Data on in vivo imaging of tumors of mice injected with BP@Hydrogel. (a) Thermal micrographs after injection with DOX and BP@Hydrogel irradiated for 5min with an 808nm laser with 1.0W/cm2 power density. Scale bar is equal to 1cm. (b) Changes of MDA-MB-231 tumor temperature in mice after exposure to a laser. Adapted from Ref. 116 with permission. (B) In vitro and in vivo IR imaging of HeLa cells incubated under the presence of BPs, BPs@Au, BPs@Fe3O4 and BPs@Au@Fe3O4 nanoparticles. (a) IR thermal images obtained after 650nm laser irradiation (with 0.5W/cm2 power density) and (b) their temperature profiles as function of irradiation time. (c) IR images and (d) corresponding temperature profiles of tumor-bearing mice injected with BPs@Au@Fe3O4 nanoparticles and a saline solution as function of irradiation time by 650nm laser with 0.5W/cm2 intensity. Adapted from Ref. 144 with permission.
Another promising nanocomposite was obtained by combining Fe3O4 and Au nanoparticles with BP sheets (BPs@Au@Fe3O4).144 This composite was tested on HeLa cells: their thermal images demonstrated that the highest temperatures were obtained after the cells were treated with BPs@Au@Fe3O4 under 650nm laser irradiation with 0.5W/cm2 power density applied for 3min (Fig. 10(B)-(a)). A very fast temperature increase around tumor location was also observed upon exposure to 650nm laser. Injection of tumors with BPs@Au@Fe3O4 nanoparticles also generated enhanced magnetic field (Fig. 10(B)-(c)). These in vitro and in vivo results were corroborated by the relevant temperature profiles recorded as function of the exposure time (Figs. 10(B) and (b) 10(B)-(d)).
Multifunctional nanosystem for bioimaging and synergistic cancer therapy purposes consisting of PEG-modified BPQDs was also developed.145 Temperature of the tumors of mice injected with PEG-modified BPQDs significantly increased (by ∼30∘C) within 2min under NIR irradiation. These results confirmed outstanding photothermal efficiency of PEG-modified BPQDs.
6.3. Fluorescence imaging
BP was also used for in vivo fluorescence imaging. One of the examples is a biocompatible delivery system consisting of BP and PEG/Ce6 nanosheets,146 which were used for guided photothermal/photodynamic cancer therapy equipped with dual-modal imaging. Two hours after the injection, fluorescent signals originated from BP@PEG/Ce6 nanosheets were detected at tumor sites (Fig. 11(a)). An increased fluorescent intensity was observed even 24h after the injection implying that BP@PEG/Ce6 accumulated in these tumors. Ex vivo fluorescence measurements of the removed mouse organs and tumors 24h after the injection showed strong fluorescence, also confirming accumulation of BP-PEG/Ce6 nanosheets in organs and tumor tissues (Fig. 11(b)).

Fig. 11. In vivo NIFR imaging: of (a) mice before and 2, 4, 8, 12 and 24h after injection with BP@PEG/Ce6 NSs; of (b) major organs and tumors of mice 24h after the injection; of (c) mice with 4T1 tumors before and after tail vein injection of BP-DEX/PEI-Cy7 and BP-DEX/PEI-FA/Cy7 nanoparticles. All graphics in (a)–(c) are adapted from Refs. 146 and 147, respectively (with permission).
Another example is in situ usage of BP nanoparticles combined with dextran (DEX) as well as branched poly(ethyleneimine) (PEI). These BP-DEX/PEI composite nanoparticles were then modified with folic acid (FA) to enhance their deposition in tumors.147 Equal amounts of these composites with (BP-DEX/PEI-FA-Cy7) and without (BP-DEX/PEI-Cy7) were then intravenously administered into mice with 4T1 tumors. The mice were then analyzed using NIR fluorescence (NIRF) at different times after the injections. Strong NIRF signal was detected coming from the whole mouse body 15min after the injection of both composites (Fig. 11(c)). NIRF intensity for both composites decreased after 15min, however, intensities of the mice injected with BP-DEX/PEI-FA/Cy7 composites was highervery likely because FA, conjugated with nanoparticles, enhanced stability of nanoparticles as well as their accumulation rate in tumors.
7. BP for Cancer Therapy
Phototherapy is a general term that covers photodynamic and photothermal therapies (PDTs and PTTs, respectively). Photothermal agents in PTT assist in localized heating, ablating cancer cells by other agents capable to absorb NIR. PDT is characterized by a sequence of photochemical reactions activated by a photosensitizer with specific light-wavelength. With regard to these two applications, BP has sparked particular scientific interest because of its outstanding carrier mobility and tunable bandgap, both of which allow BP to absorb energy in UV and visible ranges. These properties make BP-based materials excellent candidates as photosensitizers for improved PDTs and PTTs.148,149,150
7.1. Photothermal therapy
PTT typically utilizes light-absorbing compounds to cause hyperthermia (upon their exposure to NIR) to perform tumor ablation. Attention to various PTT techniques, materials and procedures significantly increased over the past several years. Unlike more common cancer treatments, such as radio- and chemo-therapy, NIR-assisted PTT has fewer side effects because flexibility associated with irradiation by light provides excellent therapeutic effects with minimal dark toxicity.151,152
Ability of BP nanomaterials to absorb over the entire visible-light region allows nano-BP to be photothermally active, which is exactly what is needed for an efficient PTT.153 For example, a PTT system consisting of BP nanosheets combined with thermosensitive hydrogel Poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) (PDLLA-PEG-PDLLA: PLEL) was used to treat cancer after the surgical removal of a tumor.154 The tumor was surgically removed through the skin incision at the edge of the tumor (Fig. 12(a)). The wound region was then treated with an 808nm laser (with 0.5W/cm2 power density), sprayed with BP@PLEL hydrogel and sutured after PTT. Temperature of the BP@PLEL group increased quickly to 39.4∘C during the initial 5s-exposure of tumor-bearing sites under NIR irradiation, which is higher than BP@PLEL hydrogel gelation point (Figs. 12(b) and 12(c)). Within 30s, the temperature rose to 58.2∘C. Mice subjected to this tumor-removal operation demonstrated a very high local recurrence rate (80%) after eight days. Average life-span of these mice was ∼35 days (Figs. 12(d) and 12(e)). These results demonstrate outstanding efficiency of BP@PLEL hydrogels during PTT.

Fig. 12. In vivo PTT treatment of cancer after the surgical tumor removal. (a) Schematic showing removal of the tumor and PTT of the surgical site. (b) IR thermographic maps and (c) time-dependent temperature change of nude mice with tumors. The mice were treated with the PLEL and BP@PLEL hydrogels and then irradiated by 808nm laser with 0.5W/cm2 power density. (d) Mice with tumors. (e) Growth curves of tumors (shown in (d)) without any treatment as well as of surgically removed tumors with and without postoperative PTT (***p < 0.001). Adapted from Ref. 154 with permission.
Another study demonstrated photothermal properties of PEG-modified BP nanoparticles139: upon their presence in the tumor, its temperature significantly increased (from 34∘C to 59∘C within 5min) upon irradiation (Figs. 13(a) and 13(b)). Mouse tumors treated with PEG-modified BP nanoparticles as well as by laser irradiation for three days shrank gradually (Figs. 13(c) and 13(d)). Tumors of all mice from the treated group disappeared, and all mice survived for >42 days (Fig. 13(e)).

Fig. 13. In vivo PTT with PEG-modified BP nanoparticles. (a) Thermal images of mice with 4T1 tumors before and 1, 3, 4 and 5min after injection of phosphate buffered saline (PBS) solution or PEG-modified BP nanoparticles (columns 1 and 2, respectively) followed by irradiation with an 808nm laser (2.0W/cm2 power density) for 5min. (b) Temperatures of mice with 4T1 tumors as function of a continuous laser irradiation time. (c) Mice with 4T1 tumors treated with PBS solution, PEG-modified BP nanoparticles and laser irradiation as well as with a combination of PEG-modified BP nanoparticles and laser irradiation. (d) Growth rates of 4T1 tumors in mice treated as described in (c). y-axis show tumor volumes normalized to their initial sizes. (e) Survival rates of mice after the treatments described in (c). Adapted from Ref. 138 with permission.
Excellent performance of BPQDs/PLGA nanospheres (NSs) during PPT was also confirmed by effective tumor ablation under NIR laser irradiation.132 MCF-7 and B16 melanoma cells were incubated with 10ppm of BPQDs/PLGA NSs for 4h and then treated with an 808nm laser (1W/cm2) for 10min. Nearly all cancerous cells were killed, which confirms outstanding PTT efficiency of BPQDs/PLGA NSs in annihilation of cancerous cells. One drawback, however, is that PTT can induce hyperthermia as well as unfavorable heat shock response from a deep-seated tumor, which, in turn, can cause inflammations, organ damage and even metastasis. Thus, to reduce or to completely eliminate these issues, additional advanced phototherapeutic strategies are needed.
7.2. Photodynamic therapy
PDT is a clinically approved technique adapted to treat several types of cancers. It is a marginally invasive novel platform with specific spatiotemporal selectivity. Unlike PTT, PDT kills cancer cells by a singlet oxygen (1O2) generated upon energy transfer from a photosensitizer when exposed to light.
An all-in-one PDT nanosystem to monitor O2 self-supply in dual-mode to enhance anti-hypoxic tumors combined with the feeding back therapy was recently reported (Figs. 14(a)–14(c)).155 A hybridized nanosystem (marked as R-MnO2-FBP) was prepared electrostatically using rhodamine B (RhB)-encapsulated MnO2 (R-MnO2) (as both O2 indicator and a supplier) with a peptide-modified BP labeled with fluorescent isothiocyanate (FITC) (as a photosensitizer and as a signal switch for cell apoptosis) treated prior to the reaction with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate(poly ethylene glycol)-2000] (PEG-FA). Upon action of mediated endocytosis coupled with FA receptor (FR), R-MnO2-FBP dissociated in H2O2-enriched and acidic environment of cancer cells, inducing in situ generation of O2 and helping to reduce hypoxia-associated PDT resistance. Both released Mn2+ and RhB demonstrated fluorescence recovery and strong T1-weighted magnetic resonance (MR) contrast, both of which are useful for fluorescence/MR dual modal imaging of the O2 supply. Duration of laser irradiation was optimized through dual-mode monitoring, after which BP-assisted PDT was realized both in vivo and in vitro. Mice irradiated 28h after intravenous injection of R-MnO2-FBP demonstrated higher contrast of cancerous formations in images obtained using RhB fluorescence with MR imaging than the group treated with the laser 24h after the injection. Thus, R-MnO2-FBP composites could deliver oxygen with dual-mode tracking (Figs. 14(d) and 14(e)). Additionally, R-MnO2-FBP-based treatment demonstrated very strong therapeutic results (Fig. 14(f)).

Fig. 14. Synthesis schematic of R-MnO2 (a) and R-MnO2-FBP (b) as well as their roles in oxygen self-supply monitoring, feedback of therapeutic effect and enhanced PDT (c). In vivo evaluation of PDT efficiency using fluorescence (d) and MR imaging (e) of mice with tumors 24, 28 and 36h after the mice were injected with R-MnO2-FBP, after which the cancers were treated by 660nm laser with 150mW/cm2 power density for 10min. Scale bar is 5.0mm. (f) Tumor volumes after different treatments (see the legend inside the graph for details). Each data point represents an average of 5 measurements. Errors represent standard deviation. Statistical analyses were performed using the Student’s test (**p < 0.01). Adapted from Ref. 155 with permission.
Huang et al. developed BP/Bi2O3 heterostructures as effective and biocompatible radiosensitizers for synergistic cancer treatment.131 These heterostructures were fabricated by growing Bi2O3 nanoparticles on BP nanosheets. In this case, Bi2O3 nanoparticles acted as inhibitors against quick BP degradation: Bi2O3 nanoparticles tend to occupy BP surface defect sites. Such synergy between BP and Bi2O3 allow 1O2 overproduction upon exposure to X-ray irradiation, which significantly improves radiotherapy results toward destruction of cancer cells through cycle-arrest and cell apoptosis.
BPQDs were also studied as potential PDT agents.156 Even though these BPQDs were very small, they exhibited excellent stability in physiological environment and no noticeable toxicity especially after these BPQDs were coupled with PEG. These BPQDs were able to generate ROS upon exposure to light. Both in vitro and in vivo studies demonstrated excellent antitumor efficiency of these BPQDs during PDT. Additionally, these small BPQDs were easily excreted by kidneys because their hydrodynamic diameters are very small. Feasibility of fabrication and implementation of dual-triggered oxygen self-supported nanosystem consisting BP nanosheets acting as both photosensitizers and nanocarriers and capable of enhancing PDT of tumors within hypoxic microenvironments were also demonstrated.157 This dual-triggered oxygen self-supply nanosystem was capable to recognize and use stimuli associated with tumor microenvironment to enhance PDT as well as to overcome resistance to the therapy often associated with hypoxia.
7.3. Synergistic therapy
Previous section described photophysical properties of BP and its ability to produce 1O2 upon irradiation by a 660nm laser for the purpose of PDT enhancement. BP-based materials also demonstrated ability to cause hyperthermia upon irradiation by an 808nm laser during PTT. However, BP biodegradation accelerates by both hyperthermia and acidic environments. Thus, BP-based materials can be used for double- or triple-response therapy upon specific targeted modifications. Because of harsh hypoxic environment of tumors, PDT efficiency is often constrained because of limited 1O2 generation. To overcome this drawback, combination of PTT and PDT was suggested. Heat produced during PTT considerably increases O2 supply and enhances blood flow and 1O2 generation. PDT significantly increases response of tumor cells to PTT by changing their microenvironment. Thus, combining PDT and PTT seem as a potentially efficient strategy for treatment of superficial tumors.
Zhang et al.158 developed genipin (GP)-polyglutamic acid (PGA)-Fe3O4-CDs@BPQDs nanocomposites to treat cancer. Photothermal activity of these composites inside the irradiated tumors were monitored by a thermal camera (Figs. 15(A) and 15(B)). Groups treated with GP-PGA-Fe3O4-CD@BPQDs and irradiated by an 808nm laser with 2W/cm2 power density showed tumor temperature increase up to ∼50∘C after irradiation for just 5min. When the same composites were used but the mice were irradiated by a 660nm laser with 0.5W/cm2 power density, tumor temperature rose to ∼42∘C after 10min irradiation. Tumor sizes and volumes after each treatment were measured by a caliper for 14 days. These results, normalized relative to the tumor original sizes, are shown in Figs. 15(C) and 15(D). It is without a doubt that free GP-PGA-Fe3O4-CDs@BPQDs nanocomposites showed an excellent therapeutic effect against cancerous tumors. Even more enhanced therapeutic effect was observed when treated by just GP-PGA-Fe3O4-CDs@BPQDs nanocomposites was combined with laser irradiation: TGI was equal to 98.8% (Fig. 15(E)). Tumors, removed 14 days after the treatments for further hematoxylin-eosin (H&E) staining, demonstrated no changes when they were treated with just PBS or with just free GP-PGA-Fe3O4-CD@BPQDs (Fig. 15(F)). In the groups, which underwent only PDT or only PTT, cell death, inflammatory cell infiltration and even cirrhosis with structural damages to the tissues were detected. In groups subjected to a combined PDT/PTT, almost all tumor cells were destroyed and were necrotic. Thus, combinational PDT/PTT therapy is superior to just single PTT or PDT because of its synergistic effect of cure.

Fig. 15. Therapeutic effects observed for mouse tumors treated with NIR after injection with GP-PGA-Fe3O4-CDs@BPQDs. (A) Highest temperature within the tumor area. (B) Thermographs of mice with tumors subjected to PTT followed by 5min irradiation with an 808nm laser with 2.0W/cm2 power density (a) and by 10min irradiation with a 660nm laser with 0.5W/cm2 power density. (C) Relative growth of tumors measured every two days. (D) Tumor growth curves. Data points represent an average of five measurements. Errors were calculated as standard deviations of the average. (E) Photographs of mice with HeLa tumors after PBS, GP-PGA-Fe3O4-CDs@BPQDs, PDT, PTT, and PDT/PTT treatments. (F) H&E staining of tumors treated as described in (E). Scale bar is 50μm. Adapted from Ref. 158 with permission.
8. BP for Drug Delivery
During recent fast-developed progress in development of various nanomaterials, a lot of them were discovered as being suitable for drug delivery and therapeutics. Considering unique properties of BP (mentioned above) with its high and very active surface area, BP-based materials are great candidates as drug nanocarriers especially in comparison to many other nanomaterials. For example, Chen and colleagues developed a drug-delivery platform based on BP nanosheets for synergetic cancer therapy.31 BP demonstrated much higher loading capability towards DOX on BP surface (equal to almost 950wt.%) comparing to other 2D nanomaterials. This BP-based system demonstrated release properties, which are pH- and photo-sensitive: drug release was accelerated under the acidic microenvironment of tumors and enhanced after 808nm laser irradiation because of photothermal characteristics of BP nanosheets. Two groups were studied: treated with just BP and with BP-DOX, respectively. Tumor temperatures increased from ∼36.2∘C to ∼53.7∘C after only 5min of irradiation, while control groups showed only increase to 41.2∘C (Fig. 16(a)). Comparison of therapeutic effects of different treatments showed that combination of PDT, BP-DOX-based chemotherapy and 660 nm laser irradiation resulted in only moderate inhibition of tumor growth (56.2%). However, combination of PTT, BP-DOX-based chemotherapy and 808nm laser irradiation demonstrated higher inhibition effect (∼84.8%, Fig. 16(b)). The most significant tumor inhibition effect was seen in tumor-bearing mice treated with a combination of BP-DOX therapy and irradiation by both 660nm and 808nm lasers: tumor growth was inhibited by 95.5% (Fig. 16(b)). Visual examination of tumor-bearing mice as well as tumors themselves after they were removed from the animals (shown in photographs in Fig. 16(c)), demonstrating that sizes of tumor treated with BP-DOX under 660nm and 808nm laser irradiations were smaller than tumors of mice from groups treated using ot her methods (Fig. 16(c)). Tissues analysis further proved that BP-DOX treatment combined with irradiation by both 660nm and 808nm lasers destroyed most of the tumor tissues, and corresponding cancerous cells became necrotic. Morphology of the cells from tumors treated using other therapy methods or their combinations remained the same (Fig. 16(d)). Meanwhile, mice did not lose body weight during the treatment (Fig. 16(e)).

Fig. 16. In vivo applications BP-DOX for tumor treatment. (a) Effects of BP- and BP-DOX-based PTT combined with irradiation by 808nm laser in comparison with tumor treatment by 808nm laser only. (b) Tumor growths after treating tumor-bearing mice with different therapy methods. Tumor volumes are reported relative to their original sizes. Each data point represents an average result for five measurements. Error bars represent standard deviations. Statistical significance (p<0.05) is marked with asterisks. (c) Photographs of the corresponding mouse tumors treated using various therapy methods. (d) Micrographs of tumor tissues stained with H&E 14 days after the treatment. After treated with BP-DOX under 660nm and 808nm laser irradiations, most of tumor tissue cells were destroyed and became necrotic, while cells partially or largely retain their normal morphology in the other groups. Scale bar corresponds to 50μm. (e) Mouse weights as function of the amount of days after the treatments. Adapted from Ref. 31 with permission.
Another study, which used BP as a delivery platform and DOX as a model drug, demonstrated similar results.26 Photothermal and therapeutic effects of BP were confirmed by the temperature increase (upon irradiation by an 808nm laser) as well as by tumor size decrease. Wang et al.159 reported a therapy platform based on combination of BP nanosheets, hyperthermia-enhanced intracellular drug delivery system and chemo-PTT. This system included paclitaxel (PTX), BP nanosheets and human serum albumin (HSA). This drug-delivery system significantly enhanced cellular uptake efficiency and antitumor therapy.
BP surface becomes negatively charged when exposed to water or high humidity, and its interlayer distance becomes ∼5.24Å.160 Thus, BP can be intercalated with small positively charged drug molecules. In fact, our recent study demonstrated functionalization of BP nanosheets by polyethylene glycol–amine (PEG–NH2), which improved BP physiological stability and biocompatibility. These PEG-functionalized BP nanosheets also demonstrated excellent theranostic properties.161 BP-PEG/Cy7 and BP-PEG-FA/Cy7 nanosheets, after they were intravenously injected into mice, distributed evenly in the mouse bodies and showed strong fluorescence 1 h after the injections (Fig. 17(a)). Twelve hours after these injections, higher fluorescent intensity was observed from tumor areas, in which BP-PEG-FA nanosheets accumulated in comparison to tumors with BP-PEG nanosheets. 24 hours after the injection, strong fluorescence signals could be observed in both types of tumor, which demonstrated efficient accumulation of PEG-functionalized BP nanosheets. In vivo experiments also showed better targeting properties of BP-PEG-FA nanosheets. Ability of PEG-functionalized BP nanosheets to in vivo accumulate in tumors and to target certain cancers were further corroborated by ex vivo analysis of organs removed 24 hours after the injection (Figs. 17(b) and 17(c)). The most clearly seen therapeutic effect was achieved for the treatment systems consisting of (a) injection of BP-PEGFA nanoparticles, NIR irradiation under the presence of chloroquine diphosphate (CQ) and of (b) BP-PEG-FA system delivering DOX as a model drug coupled with NIR irradiation under the presence of CQ (groups 6 and 7, respectively, Fig. 17(e)). Thus, the best antitumor effect from PEG-functionalized BP nanosheets was achieved by these two combinational therapies (Fig. 17(d)). No major organ tissue damage was detected (Fig. 17(f)).

Fig. 17. Results of in vivo NIR imaging of PEG-functionalized BP nanosheets. (a) Time-lapse imaging. Red dotted-line circles show tumor sites. G1 and G2 stand for Group 1 and Group 2, respectively, which correspond to BP-PEG/Cy7 NSs and BP-PEG-FA/Cy7 NSs, respectively. (b) Bioimaging of tumors and major organs 24 h after intravenous injections of PEG-functionalized BP nanosheets. H stands for heart, LI for liver, S for spleen, LU for lungs, K for kidneys, and T for tumor. (c) Distribution of BP-PEG/Cy7 and BP-PEG-FA/Cy7 nanosheets in nude mice obtained by calculating average fluorescence intensity of tumors and organs. (d) Inhibition of HeLa tumor growth upon different treatment techniques (*p < 0.05, **p < 0.01). The groups were treated as follows: Group #1 by saline, #2 by CQ, #3 by DOX, #4 by BP-PEG-FA/DOX, #5 by BP-PEG-FA+NIR, #6 by BP-PEGFA+NIR+CQ, and #7 by BP-PEG-FA/DOX+NIR+CQ. (e) Shapes of tumors extracted at the end of the tests. (f) Micrographs of H&E-stained histological tissues from major organs 14 days after the mice were treated with BP-PEG-FA NSs, BP-PEG-FA NSs+CQ and BP-PEG-FA/DOX NSs+CQ. Therapeutic response to PBS was used as a baseline. Adapted from Ref. 161 with permission.
To summarize this section, we would like to emphasize that feasibility of using BP nanosheets as drug delivery platforms was experimentally demonstrated by their excellent drug loading efficiency as well as photo-responsive drug release abilities. Drug-loaded BP-based materials can destroy cancer cells, which is especially efficient by synergetic characteristics of PTT, PDT and chemotherapy.
9. BP for Neurodegenerative Disease
Neurodegenerative diseases like Alzheimer’s or Parkinson’s are multifaceted and progressive processes, which can potentially lead to chronic illnesses.162 Thus, considering how wide-spread these conditions are, as well as mortality and morbidity associated with them, developments of advance neuropathology techniques and procedures will have an enormous economic as well as social impacts. Therefore, treatment of pathological brain conditions is one of the most demanding, complex and difficult areas in the modern medicine.163 Recent research results indicated critical pathogeny (at the beginning and throughout duration of neurogenerative illnesses) of abnormal biometal homeostasis (e.g., elevated levels of cellular cations).164 For example, Cu-dyshomeostasis is related to protein aggregation and oxidative stress.165 This oxidative stress will initiate action of cellular components (such as lipids and DNA)166 as well as influence cellular processes and signaling pathways associated with neuroprotective illnesses.167 Cu participation is critical since it provides correct pharmacological targets for treatment of neuroprotective illnesses. Taking all this into account, chelator therapy was established and confirmed to be a sensible neuroprotective strategy.168,169
Individual atoms of BP nanosheets demonstrate strong affinity relative to metal ions. Thus, BP can bind Cu2+ very effectively. Combination of this properties with excellent photothermal effect of BP nanosheets, capable to increase blood–brain barrier (BBB) permeability upon NIR irradiation because of local hyperthermia, enables BP nanosheets to act as Cu2+ concentration regulators by crossing BBB.170 It also reduces cellular ROS and protects cells from Cu2+ dyshomeostasis-based toxicity. Beneficial of photothermal effect of BP nanosheets was demonstrated by the fact that temperature of BP-free solution increased only by 3.4∘C. However, when BP were present at 20μg/mL level, temperature increased by 23∘C only 3min after irradiation (Fig. 18(a)), which was confidently attributed to BP-nanosheet photothermal effect. To confirm BBB permeability, amount of BP nanosheets transferred from upper to lower chambers was determined using in vitro BBB model (bEnd. 3-cell monolayer). Percentage of the transferred BP nanosheets increased six-fold after NIR treatment confirming that enhanced BBB permeability was induced by nano-BP photothermal effects (Fig. 18(b)).

Fig. 18. (a) Examples demonstrating photothermal effects of BP nanosheets at various concentrations. Control groups used water. (b) In vitro BBB crossing ability of BP nanosheets boosted by photothermal effect. Schematics inside the graph shows in vitro BBB model. (c) In vivo photothermal effects of different treatments: (1) treated with BP only, (2) irradiated by NIR only, (3) treated with BP and irradiated with NIR. NIR irradiation was performed using 808nm laser. (d) Photographs of mouse brains after the treatments. Discoloration is because of Evans blue dye, which was used as BBB integrity indicator. (e) NIR fluorescence of brains after treatments with (1) Cy5-PEG-BP, (2) Cy5-PEG and NIR irradiation and (3) Cy5-PEG-BP and NIR irradiation. (f) H&E staining of the main organs after BP injection. Scale bar is equal to 50μm. Adapted from Ref. 170 with permission.
To further investigate in vivo BBB crossing ability of BP nanosheets, Evans blue staining was combined with NIR fluorescence imaging. For this purpose, temperatures of mouse heads, irradiated by 808nm laser 60min after intravenous injection of BP nanosheets and Evans blue dye, was monitored by a thermal camera. Presence of BP nanosheets triggered head temperature increased to 42.2∘C. Without BP nanosheet, temperature increased only to 35.3∘C (Fig. 18(c)). To stimulate permeability of BP nanosheets, mouse head temperatures were kept at 41∘C to 43∘C for 5min, and after 24h, the brains were removed for further analysis. Blue coloration was only visible for the brain of mice injected with BP nanosheets and irradiated with NIR irradiation (Fig. 18(d)). NIR fluorescence imaging, performed on mice treated with Cy5-PEG labeled BP nanosheets (Cy5-PEG-BP), demonstrated significantly higher ex vivo and in vivo fluorescence intensity than that of the groups subjected to different treatment prior to NIR irradiation (Fig. 18(e)). Moreover, in vivo biosafety study showed no noticeable damage to mouse internal organs (e.g., lungs, heart, spleen, liver, and kidneys) caused by presence of nano-BP at a dosage of 5.25mg/kg (Fig. 18(f)). Thus, these results are yet another confirmation of great potential BP nanosheets in biomedical applications, including neuroprotective nanodrug-delivery for treatment of neurodegenerative illnesses.
10. BP for 3D Printing Scaffolds
3D printing is as ground-breaking fabrication process offering innovative solutions in variety of fields including tissue engineering,171,172 cancer therapy173 and generation of artificial organ.174 3D printing combined with advanced techniques of material design and fabrication is even capable to fabricate materials for artificial scaffolds for bone reconstruction and regeneration.175
Phosphorus is most important elements for human bones. Thus, usage of bio-compatible BP-nanosheets, containing 100% of elemental phosphorus, is a natural choice for 3D scaffold manufacturing of bone components. In fact, a very promising novel therapeutic platform for 3D printing was developed.140 This system contains BP nanosheets 3D-imprinted into a scaffold based on bio-glass (BG). The resulting material produced bifunctional BP-BG scaffolds for bone regeneration, which can potentially replace or supplement current osteosarcoma treatment strategies. End-products of BP degradation in physiological in situ environment are phosphorus-based molecules and clusters, which can significantly benefit bone regeneration process. Additionally, osteosarcoma treatments can be even more enhanced by BP photothermal properties. To assess in situ efficiency of BG-BP scaffolds, Yang et al.140 performed micro-computed tomography of removed mouse crania followed by their 3D reconstruction, which was based on density variations of osseous tissues. Their findings confirmed much better cranium repair when BG-BP scaffolds were used in comparison to when only BG-containing scaffolds were utilized (Fig. 19(a)). Spatial distribution of bone density for newly-formed osseous tissues was obtained by application of micro-CT to circular defect areas (Figs. 19(b)–19(e)). Lower bone density was found in the centers of these defects rather than at their edges (micro-CT and 3D reconstruction images shown in Figs. 19(a)–19(e)). Thus, osteogenesis occurs through osteocyte migration from the edges to the centers. Simultaneously, circular defect region shrinks, and thin newly-formed osseous tissue, spread on the interconnected pores, forms. Additionally, some residual scaffolds were still observed when BG-BP-based scaffolds were used, which indicates that decomposition of the implanted scaffolds as well as formation of new osseous tissue (which can be also referred to as osseointegration) occur simultaneously. Such positive therapeutic results were ascribed to the synergistic effects of BP nanosheets and BG scaffolds. Thus, material-guided bone regeneration process, during which osteoblasts proliferate and adhere to the artificial scaffolds initiating both osteo-conduction and -induction, is feasible. Final outcome would be formation of a completely new osseous tissue on the slowly disappearing scaffolds (because of their consumption). To analyze volumes of bones and tissues as well as bone density and porosity, histomorphometric micro-CT analysis can be used. This analysis can further demonstrate significance of BP-nanosheets as scaffold components in regulating biological signals (e.g., PO3−4) and in overall success of the therapy (Figs. 19(f)–19(h)).

Fig. 19. Experimental results demonstrating in vivo osteogenesis implementation of BP-BG-based scaffolds. ((a)–(h)) Micro-CT results. (a) 3D reconstruction of micro-CT results collected for the Sprague-Dawley rat crania (treated for eight weeks) from the osseous tissue density data. Two irregularly-shaped holes in cranial defect areas show newly-formed osseous tissues that are not thick enough to be simulated by the 3D reconstruction program. Left- and right-hand sides of the cranium were implanted with BP-BG and BG scaffolds, respectively, for direct comparison. ((b)–(e)) Micro-CT images of circular defect areas (∼5mm in diameter). Images for scaffolds made from BP-BG ((b) and (c)) and BG ((d) and (e)) were acquired using black ((b) and (d)) and white ((c) and (e)) substrates for better visualization of newly-formed osseous tissues when the color of substrates were reversed from black to white. ((f)–(h)) Bone regeneration capability of BG- and BP-BG-based scaffolds obtained from histomorphometric micro-CT. Values of bone volume/tissue volume (BV/TV, legend for the y-axis in (f)), reflect percentage of the volume of the newly-formed osseous tissue relative to entire defect area. Bone mineral density (BMD, legend for the y-axis in (g)), reflects average bone density of circular defect areas. Total porosity (h) demonstrates interior structure of newly-formed osseous tissues. Student’s criterium was applied to determine statistical differences of these parameters (shown in (f)–(h)) to index bone regeneration capability (*p < 0.05). Data shown in ((f)–(h)) are average values; errors were calculated as standard deviations. (i) CLSM of newly-formed osseous tissues six weeks after treatment with BG-BP and BG. Scale bar is equal to 200μm. Scaffolds, exposed because of the degradation, are marked by circles. They are visible for both BG and BG-BP treated groups. Surrounding newly-formed osseous tissue, migrated to the surface of scaffolds, is also seen around partially decomposed scaffold components. White triangles mark small defects occurred because of the healing process. (j) Left-hand side image: H&E staining of crania infused with BP-BG (left) and BG (right) scaffolds. Scale bar is equal to 1 mm. Middle and right-hand-side images: two cranial defects implanted with BP-BG- (middle, scale bar is equal to 400μm) and BG-based scaffolds (right, scale bar is equal to 400μm) in week 6. Red arrows show well-mineralized newly-formed osseous tissue. Black triangles show remains of the initial scaffolds. Adapted from Ref. 140 with permission.
To continue analysis of osteogenesis mechanisms and processes, newly-formed osseous tissues around BG- and BP-BG-based scaffolds were studied using confocal laser scanning microscopy (CLSM). Both types of scaffolds were visible during the degradation. At the same time, new-growing osseous tissue, surrounding the scaffolds, migrated to the scaffold surfaces. However, upon this migration, scaffolds started slowly to degrade because their constituent components were absorbed during the new tissue growth (Fig. 19(i)). Both types of scaffolds demonstrated green or red fluorescence, which implies adhesion and spreading of some osteocytes staining fluorochrome during scaffold degradation. Red fluorescence spots were surrounded by green ones, which is a demonstration of the steady bone regeneration process. Cranium staining also confirmed degradation of old scaffolds occurring simultaneously with new osseous tissue formation (Fig. 19(j)). Thus, further fabrication, analysis and implementation of 3D-fabricated scaffolds will help scientists to better understand osteogenesis mechanisms occurring in BP-guided bone regeneration process, which will advance engineering of bone tissues.
11. BP for Biosensing Applications
Concentrations of targeted biomarkers in clinical blood samples are typically very low. Thus, medical sensors with high sensitivity and specificity are needed. Because of their unique optical and electrochemical properties, BP can be used for biosensing and diagnosis of cardiovascular, cancer-related diseases. In fact, BP nanomaterials were indeed tested for electrochemical, fluorescence-based, field effect transistor (FET) and colorimetric biosensing.
One of the examples is utilization of nano-BP-based electrocatalytic tags for competitive immunoassay to detect rabbit immunoglobulin G (IgG).176 Detection was based on registering nano-impacts of BP nanoparticles and H2 release (Fig. 20(A)). Parallel experiment involved rabbit IgG marked with BP nanoparticles (Fig. 20(A)-(a)). First, tosyl-activated magnetic beads (MB) were combined with antirabbit IgG (Fig. 20(A)-(b)). To perform competitive magnetic immunoassay, the whole complex was then combined with MB/antirabbit IgG in the presence of free rabbit IgG (Fig. 20(A)-(c)). After formation of MBs/antirabbit IgG/rabbit IgG-BP complex, 15μL of this solution was drop-casted onto the screen-printed electrode, after which 15μL of 1M H2SO4 was added (Fig. 20(A)-(d)). Presence of acid led to denaturation of the protein complex and release of BP nanoparticles. Both of these processes significantly altered electrode surface (Fig. 20(A)-(e)). Detection of BP and, as a consequence, IgG content was performed using electrocatalysis-based technique: BP nanoparticles were impacted by hydrogen evolution reaction through proton reduction (Fig. 20(A)-(f)).

Fig. 20. Schematics showing nano-BP applications in biosensors. (A) BP nanosheets in electrochemical biosensors: (a, b) Formation of magnetic beads (MB) by attaching anti-rabbit IgG and tag-label of rabbit IgG to BP. (c) Incubation of MB/anti-rabbit IgG combined with rabbit IgG/BP NPs at different rabbit IgG contents. Nanoimpact-based electrochemical detection: (d) MB-based complex is placed on the working electrode; (f) detection after the impact of the liberated BP (shown in (e)). Adapted from Ref. 176 with permission. (B) BPQDs as fluorescent biosensor components: (a) synthesis of BPQDs and detection of thiols and (b) fluorescence assay to reveal AChE activity. Adapted from Ref. 177 with permission. (C) BP nanosheets as components of FET biosensors. Mechanically exfoliated BP nanosheets were placed on a device to connect to the electrodes. Probes with antibodies were decorated by Au nanoparticles. Layer of Al2O3 was applied to BP nanosheets for protection against oxidation. Adapted from Ref. 28 with permission. (D) BP applications as colorimetric biosensor components: (a) bulk BP is exfoliated to obtain several-layer BP using PVP followed by its decoration with Au particles forming BP-Au composite. BP layer was stabilized by PVP. Nano-BP served as a substrate and as a reducing agent for uniform growth of Au nanoparticles; (b) illustration of colorimetry-based immunological detection of CEA by catalytic reduction of 4-nitrophenol (4-NP) by BP-Au. Adapted from Ref. 178 with permission.
Another example demonstrates sensitive fluorescence sensing platform, not requiring any labeling, to evaluate activity of acetylcholinesterase (AChE).177 This method is based on the inner filter effects (IFE) between BPQDs and 2-nitro-5-thiobenzoate anion (TNB) (Fig. 20(B)). BPQDs were first synthesized using bulk BP as initial material by a typical top-down sonication-assisted solvothermal approach. These BPQDs exhibited green fluorescence (Fig. 20(B)-(a)). 5,5’-Dithiobis-(2-nitrobenzoic) (DTNB) Ellman’s reagent reacted with thiol groups forming TNB. Existence of IFE between BPQDs and TNB allowed development of sensitive and label-free sensing fluorescence-based platform for analysis of thiols, which was then used to evaluate AChE activity using acetylthiocholine substrate (Fig. 20(B)-(b)).
In a FET biosensing system, surface-passivated BP were utilized to detect IgG.28 such BP-based FET device is capable to detect IgG down to 10ng/mL within seconds (Fig. 20(C)). Electrochemical detection of redox-active cardiac biomarker myoglobin (Mb) using aptamer-functionalized BP nano-electrodes capable to measure direct electron transfer was fabricated. This aptamer-containing platform showed very low detection limit (equal to ∼0.524pg/mL) as well as sensitivity (equal to 36μApg−1mLcm−2) relative to Mb.29 Thus, using BP-based materials to fabricate FET biosensors is very promising for rapid diagnostics of illnesses based on detecting specific biomarker molecules and early screening of pestilence.
Optoelectronic application involving BP are also affected by its quick decomposition a degradation. However, BP instability can be very advantageous for biosensing applications. One of the examples is very sensitive and selective colorimetry developed to detect carcinoembryonic antigen (CEA) (Fig. 20(D)).178 It uses several-layer BP decorated with Au nanoparticles (BP-Au) prepared from mixture of hexachloroauric acid (HAuCl4), FLBP and water (Fig. 20(D)-(a)). This active material showed high catalytic activity towards reduction of 4-nitrophenol (4-NP). This transformation occurs with color change as the reactants convert from yellow 4-NP to colorless 4-aminophenol (4-AP). Colorimetric analysis of this reaction is efficient and accurate for the clinical detection of cancer biomarkers (such as CEA) in a wide detection range (from 1pg/mL to 10μg/mL) and with high sensitivity (0.20pg/mL) (Fig. 20(D)-(b)).
Recently, a new surface plasmon resonance (SPR) sensor based on 2D antimonene to selectively detect label-free clinically relevant miRNA-21 and miRNA-155 biomarkers was developed in our group.179 First-principles energy calculations revealed strong interaction of antimonene and ssDNA. In fact, this interaction is even stronger than that for graphene and ssNDA, which is often used for DNA detection. Such strong interaction is because of more delocalized 5s/5p orbitals of antimonene. Detection limit of antimonene-based sensor reached 10aM, which is 2.3–10 000 times higher than detection limits of currently used miRNA sensors. Attempts to combine novel sensing material with SPR architectures can unlock even more sensitive detection of miRNA and DNA as a promising avenue for cancer diagnosis, staging, and monitoring.
12. Conclusion and Future Perspective
Unique combination of properties of BP (such as strong structure-related and functional anisotropy, direct energy bandgap, excellent electron transfer characteristics, and high conductivity) make BP-based nanostructures very appealing for practical biomedical applications, such as bioimaging, cancer therapy, drug delivery, 3D printing scaffolds, and biosensing. Nevertheless, research of BP-based materials for these applications is still in its infancy especially in comparison with other well-researched 2D materials. Fabrication of BP in high quality on a large scale is still challenging. Yet, reliable and efficient large-scale production methods to obtain BP-based nanomaterials with controllable sizes, surface structures and number of layers is crucial for wide-spread biomedical applications of BP-based materials.
Preliminary, mostly fundamental, research-related results are encouraging: they demonstrate problem-free biodegradation and biocompatibility of BP. However, data is still lacking for clinical translations. Moreover, biodegradation mechanisms of BP (exact processes and reactions occurring as well as their rates) in physiological environment were not studied. To describe these mechanisms, more systematic data on BP in vivo degradation, its hemo- and histo-compatibility as well as removal pathways of BP are needed, yet they are lacking. Additionally, lasting biological effects to evaluate biosafety should also be performed to guarantee BP safety in vivo.
Moreover, surface modifications of BP are challenging because of lack of functional surface groups. Surface modification is extremely important to achieve adequate life-time of drug delivery systems upon their circulation in blood. It is also essential for drug accumulation in tumors. Certain surface modifications often require targeted functionalization, to realize which one needs to develop specific surface-engineering strategies. One of the possible routes to ensure adequate in vivo performance of BP is their surface functionalization by taking advantage of electrostatic interaction of BP surface with PEG or by coating BP sheets with regular or mesoporous silica using sol–gel technique.
In spite of these drawbacks, BP can still offer a powerful platform for multidisciplinary biomedical applications. Synergistic effects of BP-based drug delivery systems for cancer therapy were reported by many research groups. Well-established (e.g., chemotherapy) as well as just-emerging (e.g., gene- and immune-therapies) treatment methods can be supplemented and combined with nano-BP to broaden and improve their therapeutic action. BP-based treatments are not limited to only cancer treatments since some refractory diseases can be also usefully treated using nano-BP-based approaches. Some studies demonstrated use of BP-based systems as efficient neuroprotective nanodrugs for neurodegenerative diseases. Thermal effects associated with BP-presence and actions allow particles to penetrate BBB to reach the brain. Discovery of BP properties extremely useful for BP to act as efficient PDT agents and nanocarriers opened directions for new functional nanomaterial design with the goal to treat neurodegenerative and other cerebral diseases. Another example of BP application is in bio-3D printing: 3D-printed scaffold strengthened by BP can assist efficient localized treatment of osteosarcoma and bone regeneration.
Usage BP-based nanomaterials for biomedicine is still in its infancy. Detailed experimental and theoretical studies are still underway to fully uncover BP potential. It is an established knowledge that particle sizes and their modifications are critical to regulate biological behaviors of BP. However, relationships of these factors with others, e.g., biological immunity and systematic cytotoxicity, are yet to be fully understood. Additional and more detailed studies of specific BP use as well as transformation to actual clinical trials require interdisciplinary contributions from chemists and biomedical scientists as well as pharmacologists and representatives from industrial sectors. Uninterrupted development of BP-related nanotechnology will advance applications in biochemistry as well as other medical areas. Thus, one can confidently expect numerous fundamental and technological innovations in the future.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by the Joint Fund Project of Basic and Applied Basic Research Fund of Guangdong Province (2019B1515120043), the Guangdong Provincial Natural Science Foundation of China (2018A030310623), the Guangdong Provincial Medical Scientific Research Foundation of China (A2019027), the Research Fund of University of Macau (MYRG2019-00121-ICMS and MYRG2018-00207-ICMS), and the National Natural Science Fund (61435010 and 6181101252).


