Electrospun antibacterial nanofibers for wound dressings and tissue medicinal fields: A Review
Abstract
Bacterial infections are a major cause of chronic infections. Thus, antibacterial material is an urgent need in clinics. Antibacterial nanofibers, with expansive surface area, enable efficient incorporation of antibacterial agents. Meanwhile, structure similar to the extracellular matrix can accelerate cell growth. Electrospinning, the most widely used technique to fabricate nanofiber, is often used in many biomedical applications including drug delivery, regenerative medicine, wound healing and so on. Thus, this review provides an overview of all recently published studies on the development of electrospun antibacterial nanofibers in wound dressings and tissue medicinal fields. This reviewer begins with a brief introduction of electrospinning process and then discusses electrospun fibers by incorporating various types of antimicrobial agents used as in wound dressings and tissue. Finally, we finish with conclusions and further perspectives on electrospun antibacterial nanofibers as 2D biomedicine materials.
1. Introduction
Some interesting hierarchically complex structures often appear in many biological systems,1,2 and these structures endow various functionalities such as nanoscale viruses, microscale cells, millimeter skin and macroscale tissues. To date, there are various hierarchical structures used as biomedicine, such as zero-dimensional particles,3 one-dimensional fiber,4 two-dimensional substrates5,6,7,8,9,10,11,12 and even bulks.13 Among them, fibers with different sizes (nanometers or micrometers) and rich morphologies (wires or tubes) can prepare from one- to three-dimensional substrates. A series of approaches have been developed for fabricating fibers such as melt spinning, solution spinning, chemical vapor deposition, sinter technology, electrospinning technology and so on. Electrospinning technology is a simple and cost-effective method for developing micro-nanometer continuous fibers from plenty of polymers or compounds.14,15,16,17 The prepared electrospun fibers possess small size, large specific surface area and high porosity, which have a wide application in biomedical fields, such as wound dressings, tissue engineering, regenerative medicine and so on.
As we all know, bacterial infections have been a primary cause of chronic infections in the world.18,19,20,21 Skin, as the primary function of human being to protect muscles, bones and internal organs, is usually wounded by cuts, burns or illnesses.22 Therefore, the wound-healing process would start immediately when the skin is injured. Once the microbial invasion starts, the skin wound-healing process became slow and even infections as well. Gram-positive organisms such as Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes) would engender immediately. If these gram-positive organisms could be killed in time, the wound recovers faster. Furthermore, some exposed tissue is also infected easily after injury and surgery, which may cause disease aggravation and even death. Therefore, it is essential to fabricate antibacterial materials that can prevent bacteria entering into the wound. To achieve this, a series of materials with intrinsic bactericidal activity or some biomaterial incorporating antimicrobials are being developed. Antibacterial nanofibers, as a relatively new class of antibacterial materials, have emerged that offer distinct advantages. Antibacterial nanofibers comprising many intersecting nanofibers with micro-nanometers can provide a notably large specific surface area and porosity and, similar to the cell extracellular matrix structure, will benefit for the cell growth. Most significantly, compared with other antibacterial materials, the expansive surface area of antibacterial nanofibers enables efficient loading of antibacterial agents. Thus, it shows a promising potential for antibacterial biological areas.
Herein, an overview of the electrospun antibacterial nanofibers incorporated in wound dressings and tissue is provided. Furthermore, the recent developments in fabrication of antibacterial nanofibers for the purpose of wound dressings and tissue engineering are discussed in detail.
2. Electrospinning Process
The electrospinning process is divided into melt electrospinning and solution electrospinning. Melt electrospinning is relatively under-investigated compared with solution electrospinning because of the complex experimental equipment, poor polymer types and large size. In this paper, the electrospinning solution is only introduced because of the wide application area and simple process.23,24 The essential components of electrospinning process are: high voltage power supply, spinneret and collecting plate such as a metallic screen, and 3-plate or rotating mandrel (shown in Fig. 1).
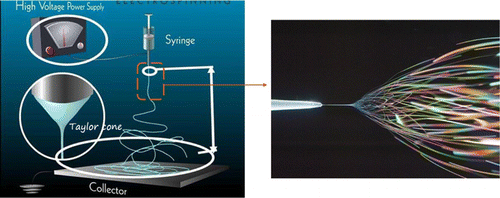
Fig. 1. Schematic of solution electrospinning process.
In brief, polymer solution is drawn using a high voltage generation. When the voltage increases, the solution at the tip of the capillary elongates and forms a Taylor cone. With further increasing the applied voltage, the charged jet would be rejected from the Taylor cone reaching the collector. Dry electrospun nanofibers randomly spread on the collector with the solvent evaporating. During the electrospinning process, several factors would affect the fiber morphology.25,26,27 These factors can be divided into four categories: polymer solution parameters, process parameters, spinnerets and environmental parameters. The solution parameters are the solvent type, polymer molecular weight, solution concentration, solution viscosity, solution conductivity, surface tension and so on. The process parameters include the applied voltage, flow rate, the distance between the needle tip and collector, etc. The spinnerets contain needleless, single, coaxial, side-by-side, tri-axial and four-axial which would generate different structural nanofibers (shown in Fig. 2).28 Environmental factors include relative humidity, atmosphere and temperature. All the parameters affect the morphology of the electrospun nanofibers. Thus, it is essential to optimize the electrospinning process factors for fabricating the suitable nanofibers.
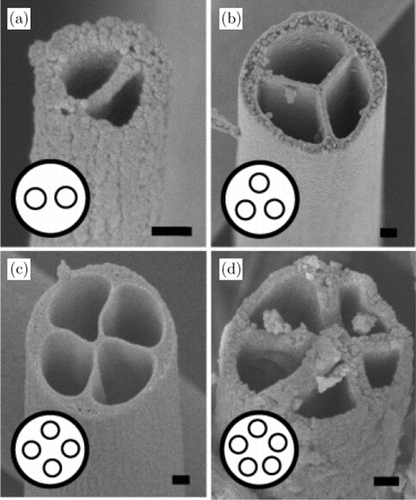
Fig. 2. SEM images of nanofibers with different spinnerets. Scale bars are 100nm.
3. Electrospun Antibacterial Nanofibers for Wound Dressings
Wound dressings are helpful to prevent infection and maintain an appropriate wound environment for healing.22,29 Up to now, there are various antibacterial nanofibers developed in wound dressings. Different antimicrobial agents were introduced into nanofibers to improve the antimicrobial properties.
3.1. Antibiotics
Antibiotics are the major antimicrobials used in clinic due to their excellent antibacterial activity. Currently, there are aminoglycosides,30 beta-lactams,31 glycopeptides,32 quinolones,33 sulfonamides34 and tetracyclines35 used to produce wound dressings. Shen Chao reported the tigecycline-loaded sericin/poly(vinylalcohol) (TC-SS/PVA) fibers prepared via electrospinning technology.36 The average diameter TC-SS/PVA fibers was 356±5.98nm and the surface of fibers was smooth. Meanwhile, the TC encapsulation efficiency in TC-SS/PVA composite fibers was up to 84.25%. The TC release amount reached 75% after 100min from TC-SS/PVA composite fibers. More importantly, the TC-SS/PVA fibers had satisfactory antibacterial activity and the inhibition zone was 40.51mm and 30.13mm against Bacillus subtilis and Escherichia coli, respectively. The results show that TC-SS/PVA fibers have a potential application for wound dressing. Heyu Li prepared hydrophilic poly(vinylpyrrolidone)/ethyl cellulose (PVP/EC) nanofibers via electrospinning.37 Ciprofloxacin (CIF), as a model antibiotic, was also loaded into the PVP/EC nanofibers forming PVP/EC/CIF nanofibers. The diameter of PVP/EC/CIF nanofibers decreased to 541±162nm, compared with the PVP/EC nanofibers (832±241nm). About 90% of CIF was loaded into the PVP/EC/CIF nanofibers. There was a CE initial burst release (7%) for 4h and a communicative release (50.0±4.4%) over 50h. A clear inhibition zone around PVP/EC/CIF nanofibers was observed after 24h against both the Gram-positive and Gram-negative bacteria. The inhibition zones of the PVP/EC/CIF nanofibers were 5.30cm and 5.71cm for E. coli and S. aureus, respectively. This study demonstrates that PVP/EC/CIF nanofibers are promising as wound dressings.
3.2. Metal nanoparticles as antimicrobial agents
Metal nanoparticles are often supposed as antibacterial agents due to their good antimicrobial properties. Among them, silver nanoparticles exhibit outstanding antibacterial activity against S. aureus bacteria and E. coli. Davood et al.38 proposed an antibacterial electrospun polyacrylonitrile (PAN) nanofibrous membrane through a surface functionalized with silver nanoparticles (AgNPs). The diameter of PAN-AgNPs nanofibers ranged 279.25±42.94 to 293.37±54.65. The max cumulative silver release was 7∗10−5% with 72h. Furthermore, the PAN-AgNPs nanofibers could kill E. coli and S. aureus bacteria. Notably, the kill ability of PAN-AgNPs nanofibers was different. The diameter inhibition zone of the PAN-AgNPs nanofiber increased from 15mm to 21mm with the increase of AgNPs content to the E. coli aureus and the inhibition zone maintain 22mm with different contents AgNPs to S. aureus bacteria. Augustine et al. also developed novel polyvinyl alcohol (PVA) nanofibers with silver nanoparticle (AgNPs) by electrospinning technology for wound dressing applications.39 No beads or irregularities were observed on PVA-AgNPs nanofibers and the average fiber diameter was only 403nm. The fabricated PVA-AgNPs nanofibrous membranes showed good antibacterial activity against both S. aureus and E. coli.
3.3. Natural antibacterial agents
Nowadays, some antibacterial agents from natural sources are also introduced to nanofibers to increase their antimicrobial activity.40 Kuntzle et al.41 evaluated chitosan/polyethylene oxide (CS/PEO) composite nanofibers loaded nature microalgal phenolic via electrospinning technology. Nanofibers produced by electrospinning solution (3% CS/2% PEO/1% phenolic) owned an average diameter of 214±37nm. The antibacterial activity of the nanofibers was also high. The inhibition zone was 6.4±1.1mm and 5.5±0.4mm for S. aureus and E. coli, respectively. Bui et al.42 fabricated polycaprolactone (PCL) nanofibers-loaded curcumin (Cur) and polyethyleneglycol (PEG) using electrospinning. The average diameter of Cur-loaded PCL nanofibers was 553nm and the nanofibers were uniform, distribution from 300nm to 1200nm. The average diameter increased to 680nm with Cur loaded and many pores appeared on the surface. Both the PCL and PCL-PEG nanofibers containing Cur exhibited good antibacterial activity against S. aureus. The wound closure rate (Fig. 3 shown) also demonstrated that Cur-loaded PCL-PEG nanofiber could facilitate wound healing faster.
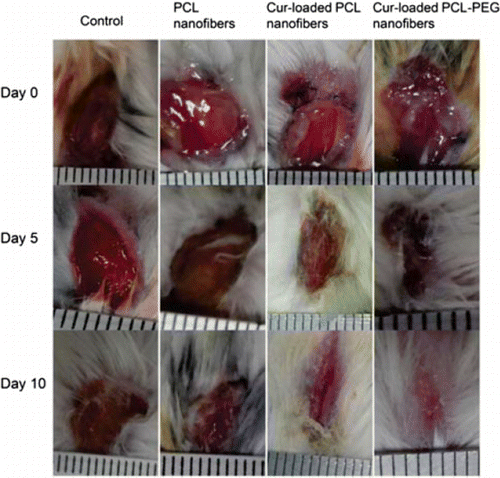
Fig. 3. Photograph of wound closure rate of mouse dorsal for nanofibers treated on days 0, 5 and 10.
3.4. Dual antibacterial agents
In order to satisfy some special disease treatments, such as the long-time sustained antimicrobial activity and short-time explosive antimicrobial activity, nanofibers with dual antibacterial agents were also studied. Yang et al.43 prepared ethyl cellulose (EC) and polyvinylpyrrolidone (PVP) nanofibers via side-by-side electrospinning. Meanwhile, silver nanoparticles (AgNPs) and ciprofloxacin (CIP) were loaded in two sides. The average diameter of these PVP/EC nanofibers loading AgNPs and CIP was 0.78±0.18nm. Over 90% of CIP was released within the first 30min, demonstrating good antibacterial activity at the wound healing initial stages. Meanwhile, the inhibition zones against S. aureus and E. coli are 19.3±0.3mm and 24.0±0.3mm after 48h, respectively. This side-by-side PVP/EC nanofiber had excellent antibacterial action whether short time or not long time. The PVP-CIP side could provide a powerful initial antibacterial effect with the CIP immediate release, and the EC-AgNPs side possessed a long-time sustained antibacterial action. Han et al.44 proposed poly(lactic acid) (PLA) nanofibers containing tetracycline hydrochloride (TCH) and tetracycline hydrochloride/carboxylated Fe3O4 (TCH/Fe3O4-COOH) nanoparticles via electrospinning technology and electrostatic interaction. The average diameters of PLA, Fe3O4-COOH/PLA, TCH/PLA and TCH/Fe3O4-COOH/PLA nanofibers were 609±103, 564±72, 466±71 and 42±68nm, respectively. The diameter of inhibition zone of Fe3O4-COOH/PLA, TCH/PLA and TCH/Fe3O4-COOH/PLA nanofiber membranes was 13.6±1.2, 17.2±1.3 and 25.8±1.4mm against S. aureus, respectively. The inhibition zone was 14.2±1.5, 16.1±1.6 and 23.6±1.6mm against E. coli, respectively (shown in Fig. 4). This result represented that TCH/Fe3O4-COOH/PLA nanofibers showed stronger antibacterial activity against E. coli and S. aureus. The improved antibacterial activities will exploit new applications as wound dressings.
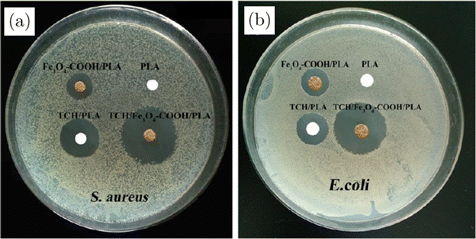
Fig. 4. Antimicrobial activities of PLA, Fe3O4-COOH/PLA, TCH/PLA and TCH/Fe3O4-COOH/PLA electrospun nanofiber membranes against S. aureus and E. coli.
4. Electrospun Antibacterial Nanofibers for Tissue Engineering
Tissue engineering, as a promising method, could improve effectively the health and life quality. The main purpose of tissue engineering is to fabricate the artificial tissue for displacing the biological functions in tissue regeneration.45,46,47 There are many materials for improving tissue regeneration, such as membranes, films, gels, etc. Electrospun nanofibrous membrane is the best one because of some unique properties such as the high surface area to volume ratio, porosity and morphological similarity to the extracellular matrix. Up to now, there are many applications for tissue engineering. Liu et al.48 proposed novel poly(L-lactide-co-caprolactone) (PLCL) nanofibers with excellent mechanical properties via the core-spun electrospinning technology, and then they could be used to fabricate small diameter (1.5mm) PLCL/tussah silk fibroin (TSF) vascular scaffolds. This artificial blood vessel could effectively promote proliferation and vascular endothelial cell adhesion as vascular scaffolds. Balagangadharan et al.46 reviewed chitosan (CS)-based electrospun nanofibers in bone tissue engineering. This reviewer provided a detailed study available on CS electrospun nanofibers for bone tissue engineering with associated excellent properties. In tissue engineering, antimicrobial is the same. Currently, there are several ways to improve the antimicrobial as follows:
4.1. Antibiotics
Ding et al.49 reported electrospun polyhydroxybutyrate/ poly(ε-caprolac-tone) (PHB/PCL) and PHB/PCL/sol–gel-derived silica (SGS) hybrid scaffolds containing antibiotic levofloxacin (LFX) via electrospinning technology. The diameter of PHB/PCL and PHB/PCL/SGS fiber was 0.8±0.2μm and 0.8±0.1μm, respectively. The tensile strength was 3.5±0.1MPa and 2.4±0.1MPa, respectively. The bacterial inhibitory activity of PHB/PCL and PHB/PCL/SGS scaffolds was evaluated using Gram-positive bacteria S. aureus and Gram-negative bacteria E. coli. The inhibition zone was 0mm and 43±2mm for HB/PCL and PHB/PCL/SGS scaffolds against S. aureus, implying that PHB/PCL/SGS scaffolds are able to effectively restrain the bacterial growth. Meanwhile, only few colonies can be observed in the culture plate against E. coli, suggesting that the PHB/PCL/SGS scaffolds have a stronger inhibitory effect on E. coli bacteria than S. aureus bacteria. Pierchala et al.50 also proposed a multilayered polylactic acid (PLA)/halloysite (HAL) porous nanofibrous membrane developed by electrospinning technique. The PLA/HAL electrospun nanofibers represented smooth and uniform. The multilayered PLA/HAL porous nanofibrous membrane included three layers (shown in Fig. 5): layer 1, different average diameter 0.27±0.08μm and 5.26±1.46μm staggered to form fibrous membranes; layer 2, porous PLA fibers loaded with HAL and HAL was distributed both inside pores and on the surface; layer 3, smooth PLA fibers incorporated with HAL had an average diameter of 1.09±0.25μm. The tensile stress of PLA/HAL nanofibrous membrane was 7.26±0.66MPa. The inhibition zone of PLA/HAL nanofibrous membrane was 16mm and 30mm for S. aureus and E. coli, respectively. These results demonstrated that PLA/HAL multilayered nanofibrous membrane had excellent antimicrobial activity with excellent mechanical and antimicrobial property.
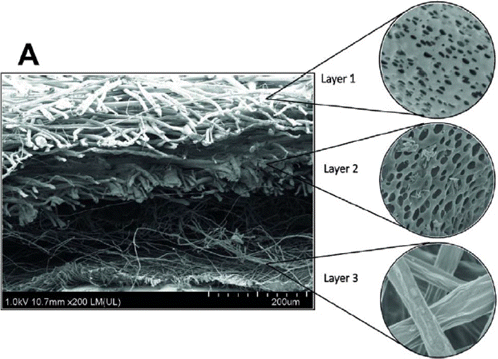
Fig. 5. FESEM micrograph of multilayered polylactic acid (PLA)/halloysite (HAL) porous nanofibrous membrane.
4.2. Natural antibacterial agents
Cur, a natural drug obtained from the rhizomes of turmeric, possesses excellent antibacterial, anti-inflammatory, wound-healing and anticancer activity. Roya et al.51 synthetized Zn-curcumin complex (Zn-Cur) and loaded polycaprolactone (PCL) core electrospun solution as a bioactive biomolecule. Meanwhile, graphene oxide (GO), another bioactive biomolecule, loaded into bioactive (PVA) shell electrospun solution. Core–shell nanofibers were prepared successfully by coaxial electrospinning technology, and the property was systematically investigated for bone regeneration. The electrospinning process of core–shell nanofibers is represented in Fig. 6. The diameter nanofibers could be controlled by electrospinning process parameters ranging 153±31nm to 205±92nm. The tensile strengths of Zn-Cur containing nanofibers with 2wt.% of GO could achieve 29±2.5MPa. For Zn-Cur containing nanofibers and drug-free nanofibers, the inhibition zone against S. aureus was around 30±1mm and 25±2mm, respectively. Meanwhile, the zone of inhibition was 26±2mm and 19±1mm for E. coli, respectively. Antibacterial test results directly indicated that nanofibers containing Zn-Cur could kill these organisms. Rad et al.52 made porous scaffold by electrospinning technology. Corn protein (Zein), polycaprolactone (PCL) and gum arabic (GA), as the main materials, were used to simulate a skin scaffold with extracellular matrix. The average diameter of PCL/Zein/GA nanofiber was 410.4±258.9nm and the tensile strength was 1.87±0.34MPa. The inhibition rings of PCL/Zein/GA scaffold against gram-negative bacteria were observed. These results indicated that this composite nanofiber had excellent antibacterial property used in wound healing. Myriam et al.53 also reported poly-ε-caprolactone (PCL) scaffolds via electrospun and 3D printed and functionalized by natural methacrylic acid N-hydroxysuccinimide ester. The antibacterial properties were excellent against S. aureus ATCC 6538 and Staphylococcus epidermidis.
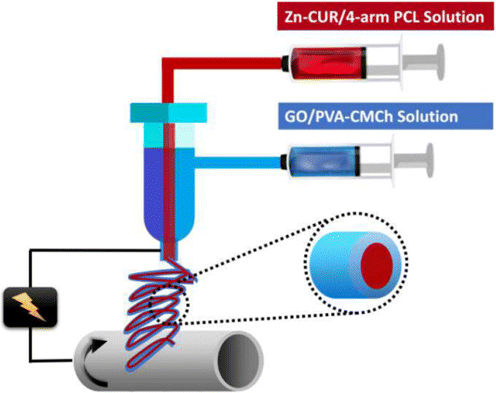
Fig. 6. Electrospinning process of containing core–shell nanofibers.
4.3. Growth factors and proteins
Mesenchymal stem cell is very important in many biomedical engineering studies. Mehdi et al.54 prepared dendriplexes (DPs) of generation 4 polyamidoamine (G4-PAMAM)/bone morphogenetic protein-2 (BMP-2) plasmid. Poly-l-lactic acid/poly(ethylene oxide) (PLLA/PEO) and poly-l-lactic acid (PLLA) scaffolds loaded hydroxyapatite nanoparticles (HA) and DPs were prepared by electrospinning in order to provide stem cell growth and differentiation environment. The diameter of PLLA/PEO/HA/DP nanofiber was 895nm. In addition, the tensile strength was up to 1.3MPa. HA and DPs incorporated in PLLA/PEO/HA/DP scaffolds would enhance osteogenesis and had a synergistic effect and expression ALP, OPN and BMP-2. Qian et al.55 fabricated a novel silver-modified/collagen-coated electrospun poly-lactic-co-glycolic acid/polycaprolactone (PLGA/PCL) scaffold, named as PP-pDA-Ag-COL, to overcome the limitations of current scaffold osteogenic and antimicrobial properties. PLGA/PCL nanofibers matrix was generated by electrospinning, and silver nanoparticles (AgNPs) also were impregnated via in situ reduction. Finally, polydopamine coats on the surface of PLGA/PCL fibers to make collagen I coat. The average diameter of PP-pDA-Ag-COL scaffold was 477±186nm and the Young’s modulus of PP-pDA-Ag-COL was 30.5±12.6MPa. Meanwhile, PP-pDA-Ag-COL scaffold represented excellent antibacterial property. The antibacterial zone was 12.51±0.1mm DIZ for S. aureus and 7.52±0.1mm DIZ for Streptococcus mutans. All results showed that this novel PP-pDA-Ag-COL scaffold possessed biocompatibility and outstanding antibacterial properties, which have potential in the craniofacial bone regeneration area.
4.4. Nanoparticles
Kang et al.56 developed a tissue-engineered trachea, containing multilevel polylactide (PLA) nanofibrous membranes with electrospinning technology and thermoplastic polyurethane (TPU) skeletons with 3D-printed. Ionic liquid-functioned graphene oxide (GO-g-IL) was composed and loaded in the PLA membrane. The tissue-engineered trachea is presented in Fig. 7. The PLA membrane represents a tensile stress of 4.31±0.67MPa. The tensile strength increased to 8.41±0.75MPa with the addition of GO-g-IL. Meanwhile, a sharp decrease was observed to E. coli and S. aureus half maximal inhibitory concentration (IC50) after adding GO-g-IL. For E. coli, the IC50 of PLA/GO-g-IL was only 0.8μg/mL compared with PLA/GO (55μg/mL). For S. aureus, the IC50 of PLA/GO-g-IL was 0.76μg/mL, far lower than PLA/GO (48μg/mL). Furthermore, the PLA/GO-g-IL fibrous membranes had excellent mechanical properties and antibacterial effects against E. coli and S. aureus cells, which would be used in tracheal repair or transplant application. Jin et al.57 also fabricated a novel silver ion-loaded calcium phosphate/chitosan (Ag-CaP/CS) nanofibrous membrane via a one-step method called electrospinning. The Ag-CaP/CS nanofibrous membrane was biomimetic and bioactive. After the addition of CaP, the surface of CaP/CS nanofibers becomes rougher than CS fibers.58 The diameter of fibers was with a broad range from 0 to 1000nm. Serried Porphyromonas gingivalis bacteria attached to the CS and CaP/CS fibers. However, only a few bacteria could be seen on the Ag-CaP/CS fibers. These results indicated that the Ag-CaP/CS fibrous membranes show a strong antimicrobial property and have great potential in guided bone regeneration.
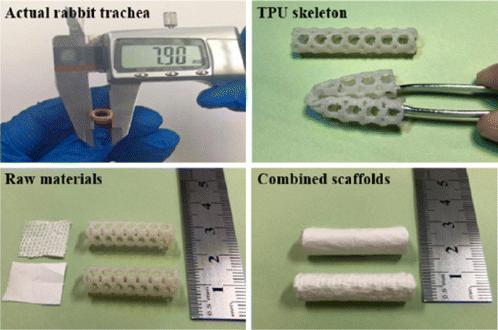
Fig. 7. Photograph of the actual trachea of rabbit and the raw materials of combined scaffolds.
5. Conclusion and Future Perspective
Researchers had developed electrospun nanofibers with compositions and architecture wound dressing and tissue engineering. The electrospun nanofibers possess some unique properties, such as large specific surface area, high porosity and morphological property similar to the extracellular matrix, which will benefit to their fabrication into wound dressings tissue engineering.
Different antimicrobial agents are incorporated into electrospun nanofibers for prevention of infection. Although they have a very significant role in antibacterial property, bacteria develop resistance to most antimicrobial agents especially antibiotics. Thus, the antimicrobial electrospun nanofibers should be designed to the smart antimicrobial. There are two channels to achieve it. One is fabricating the smart antimicrobial electrospun nanofibers according to wound microenvironment. Temperature, pH, oxygen content will happen to change with wound healing. Smart antimicrobial electrospun nanofibers should be designed according to these changes of microenvironment. Another way is fabricating the smart antimicrobial electrospun nanofibers, which can control the antimicrobial through the external conditions. This smart one would have ability to not only store the antimicrobial agents when the antibacterial properties are not needed but also release the antimicrobial agents in time with antibacterial performance required.
Furthermore, the antibacterial efficiency should be improved further. Some novel polymers with antibacterial effect group should be designed and synthesized, which not only has self-antibacterial effect, but also can add another antibacterial factor. More importantly, this antibacterial material would remain as sustained antibacterial effect for long time.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Project No. 51573103, No. 21274094) and 2019 Foundation Research fostering project 21 and postdoctoral fund (2019SCU12007) from SiChuan University.
Conflict of Interest
The authors have no conflicts of interest relevant to this article.


