Ultrabright bimetallic AuAg complex: From luminescence mechanism to biological application
Abstract
Metal clusters have attracted wide interests due to their unique electronic and optical properties, but the low luminescence quantum yield (QY) prevents them from potential biomedical applications. In this work, silver-doped Au nanoclusters (NCs) are shown to be able to improve the QY of metal clusters. We succeeded in synthesizing ultrabright glutathione (GSH) protected AuAg clusters with 10.8% QY by a one-pot route. Their florescence is about 7.5 times brighter than pure Au NCs, with super photostability and good biocompatibility in physiological environment. Based on density functional theory (DFT) calculations, we investigated the electronic structures and optical properties of the AuAg NCs. The results show that the increase of the density of states of the lowest unoccupied molecular orbital (LUMO) leads to the fluorescence enhancement. In addition, two-photon excitation fluorescence imaging has been performed to show their great potential for biomedicine.
1. Introduction
In recent decades, nanoclusters (NCs) have been widely studied for their well-defined atomic structures,1,2,3 excellent optical properties and good biocompatibility for biomedical applications.4,5,6 One of the most prominent NCs is Au NCs by virtue of their outstanding properties in bioimaging. For instance, the ultrasmall Ag-doped Au NCs at scale of Fermi wavelength show semiconductor-like strong fluorescence,7,8,9,10,11 excellent stability for anti-oxidation and less reactivity towards oxidation agents,12 which are crucial for biological applications.13 A series of research reveal that many factors affect fluorescent properties, including the structure of NCs,14 size effect,15 spatial position of doped metal atoms,2 the interlocking between kernel-surface and surface ligands.3 Above all, ligand properties are the most significant on enhancing fluorescence and developing other functions.
Typical hydrophilic ligands employed to synthesis16 are polymer microgels, nanotubes, DNA, peptides,17 and thiolate (e.g., glutathione (GSH),4,18 dihydrolipoic acid (DHLA),19 dithiothreitol (DTT)). Meanwhile ligands are critical to prevent core metal core from aggregating or etching, so that they determine the stability of the clusters. Among which, glutathione (GSH) is the most appealing. As both reducing agents and ligands, they could coordinate to Au atoms to form considerably stable Au–S bonds to against core etching18 as well as possesses excellent thermostability and photostability, compared with other ligands.20,21 There is a feasible method to synthesize Au complex based on the previous researches.22,23 Theories of the luminescence of NCs has been proposed to reveal the intrinsic mechanism, such as free electron model24 and aggregation induced emission (AIE).25,26 Furthermore, the latest research indicates potential mechanism, such as ligand-to-metal charge transfer (LMCT) and ligand-to-metal-metal charge transfer (LMMCT) has been proposed to interpret interior principles of luminescence. However, it is still having barriers for taking NCs into biomedical applications, because of their low quantum yield (QY) and poor photostability. Therefore, it is desirable to exploit ideal NCs to overcome these disadvantages.
Metal doping is another effective method to improve fluorescence properties of NCs.27,28 Ag, Zn,29 Cd,30 Cu and other metallic doping have been used to improve luminescence brightness of metal NCs.31,32,33,34 Compared with Au atoms, outer electrons of Ag atoms have smaller effective mass owning to less sd hybridization, and better optical properties due to larger electron mobility.35 Discrete ligands (such as PEG series, GSH, peptides) capped AuAg NCs have been widely reported to enhance the QY by extending the chains of ligands and introducing sulfur/nitrogen atoms.30 In addition, Ag doping could also improve intracellular stability of NCs and decrease the interactions between biomolecules and NCs.30 Lifetime of available fluorescence NCs is interesting, which is closely related to optical transition and luminescence mechanisms of NCs. Previous extensive studies demonstrated that owning to the doping of Ag, optically active levels could be split, which causes some forbidden transitions in the interaction of Au-Ag.36 Moreover, Ag doping might yield charge traps37 to extend the lifetime of fluorescence. According to above theories, Ag doping has great potential in improving optical performance of NCs.
In this work, we present a new series of GSH-protected Ag-doped Au NCs with high quantum yield (∼10.8%), excellent photostability and a 7.5-fold luminescence enhancement. The NCs are synthesized by standard wet chemical synthesis. GSH (–SG) ligands, including electron-rich atoms (like O and N atoms) and electron-rich groups, could enhance fluorescence intensity12,38 and ensure fine biocompatibility1 by strengthening the interaction between core atoms and surface combinations. Structure and optical properties of this new series of Au NCs are investigated in detail. Based on DFT calculations, we attribute the fluorescence enhancement to the extra optical transitions between the electronic states of Au NCs induced by Ag doping.
2. Materials and Methods
2.1. Chemicals and synthesis
The synthesis of bimetallic AuAg clusters references to the previous reports but with a modification.25 To synthesize different doping ratio AuAg NCs, for example, 0.8mL chloroauric acid (20mM, Aladdin) and 0.2mL silver nitrate (20mM, Aladdin) were mixed in a flask with stirring till a yellow and clear solution had been formed. Then, 0.3mL glutathione (100mM, Heowns) was added to the flask. After that, 8.7mL deionized water was dropped slowly in the resulting solution. Finally, the mixture was left heated at 70∘ by oil-bath while smoothly stirring (500rpm) for 24h. The bright golden solution could be obtained after above procedures. The solution was collected by 10k and 30k ultrafiltration tubes (MWCO). The final collection was washed several times with deionized water and filtered by 3k ultrafiltration tubes. A slight fade could be noticed after purification. To determine the definite concentration, for example, in toxicity test, solid powder of NCs needs to be prepared by freeze-dried. The essential fetal bovine serum (FBS) and phosphate buffered saline (PBS) in stability measurements purchased from Gibco and Solarbio and PBS were pre-adjusted to the pH to 7.4 using hydrochloric acid.
2.2. Characterization
Morphologies of as-prepared sample were studied using TEM, HRTEM and elements mapping was measured with JEM-2100F microscope (JEOL) operated at 200kV. Fluorescence spectra were recorded with Hitachi F-2500 fluorescent spectrophotometer and performed at room temperature (295K) in a quartz fluorescent cuvette with 1×1cm in size. The spectra were recorded at the excitation wavelength=375nm. The slit width was 5 nm. Surface combinations and valence states of elements were detected by PHI XPS (Perkin Elmer) with the Al Kα excitation. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) was performed by Bruker Autoflex III TOF/TOF200. UV-Vis spectroscopy measurements (300–700nm) are performed by UV-3600 spectrophotometer (Shimadzu). Fourier transform infrared spectroscopy (FT-IR) was analyzed with AVATAR 360 (Nicolet Instrument). Fluorescence lifetime was measured by FLS920P (Edinburgh Instrument). Fluorescence spectra was measured by Fluorolog3 (Horiba). All above instruments supported by Tianjin University.
2.3. Quantum yield calculation
We have calculated QY of AuAg NCs in a certain concentration in aqueous phase. Here, an accessible fluorescent dye of known QY as reference is essential, so that we chose Cy5 as reference (20% of QY in aqueous solution). We measured absorption spectra of as-prepared NCs and references as well as their fluorescence spectra, with excitations at 365nm and 650nm, respectively. The calculation formula is as follows39 :
2.4. Biodistribution imaging, hematology and biochemistry examination
These analyses were carried out by standard saphenous vein blood collection technique with potassium-EDTA collection tubes. 1mL of mice’s blood was collected via vein after treated with intraperitoneal injection of certain time, and then separated by centrifugation and fraction. Then, the mice were sacrificed by isoflurane anesthetic and angio catheter exsanguinations, and major organs from those mice were harvested, fixed in 10% neutral buffered formalin, processed routinely into paraffin, stained with H&E and examined by a digital microscope. This examination was carried out strictly in accordance to the Chinese Academy of Medical Sciences (CAMS) guidelines for the care and use of laboratory animals and was approved by Animal Care and Use Committee (IACUC), and handled under the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (Tianjin, China).
2.5. 2PM imaging
This experiment was carried out by Nikon A1R MP two-photon excitation fluorescence microscopy. Balb/c mice were chosen to had AuAg NCs (18mg/mL, 0.2mL) by intravenous injection. After 2h, mice had a skull operation which opened a hole (∼2mm). Then, we attached the mice on the platform and adjusted the focal of the objective lens (Nikon 40×0.8NA) in bright field to fix a position, turned off the bright field light and switched on the excitation laser to 800nm excitation simultaneously. Finally, we opened the red channel and adjusted to proper laser power when the vessel is in vision.
3. Result and Discussion
We found that the optical properties of our bimetallic AuAg NCs are highly sensitive to the doping concentration. UV-Vis absorption spectrum shows a weak but significant broad “shoulder” centered at about 405nm (Fig. 1(a)) in the near ultraviolet range, due to sp-sp (intra-band) and d-sp (inert-band) electronic transitions. It should be noted that no significant surface plasmon resonance (SPR) peaks are found owing to the strong quantum size effects of clusters below 5 nm, the absence of SPR peaks, which usually at 500–550nm, indicates the formation of NCs rather than nanoparticles (NPs). And such properties are remarkable distinctions between ultrasmall Au clusters and Au NPs.8,40 These spectra are similar to another thiolate-protected AuxNCs (x<25).41,42 The excitation and emission spectra show a symmetric emission peak at 606nm under the excitation of ultraviolet (375nm) as shown in Fig. 1(b). We modulated the silver doping proportion and changed the ligand ratio during synthesis in solution, but found no obvious changes in the absorption spectra (Figs. 1(c) and S1), which is in consistent with previous studies of AuAg NCs.43 Interestingly, when we tried different molar ratio on Ag doping, the fluorescence intensities are enhanced dramatically, implying silver atoms are predominant in luminescence enhancement. It can be seen that an enhancement as large as 7.5-fold can be achieved at the doping ratio of 18% compared with pure Au NCs, at the excitation of 375nm (Figs. 1(d) and S2). We found a subtle blue-shift at the emission peak under the same excitation wavelength due to the doping effects. The QY of AuAg NCs is about 10.3% (Fig. S3), significantly brighter than the reported homogeneous pure Au clusters such as Au25SG18 (less than 0.1%),2 which indicates the great potential of Ag-doped Au NCs for bioimaging. In addition, we explored the fluorescence intensities in various reaction time. The results showed that 24h is the optimized reaction time with maximum fluorescence intensity (Fig. S4). In addition, AuAg NCs exhibit ultrahigh stability and fluorescence intensity lasting for 2 weeks with negligible decay (Fig. S5) and without any deteriorations.
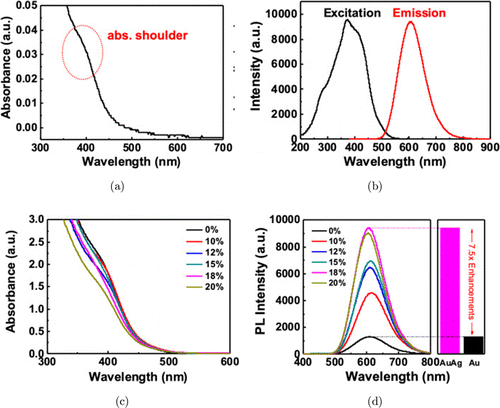
Fig. 1. Optical properties including (a) UV-Vis spectrum of freshly prepared GSH-protected AuAg NCs. The weak characteristic absorption shoulder can be found at ∼405 nm, (b) excitation and emission spectrum, there is a stokes-shift of 235nm, (c) absorption spectra with varied silver doping ratio from 0 to 20%, there are no obvious different between these curves and (d) fluorescence spectra under same concentration of different doping scales, 7.5-fold fluorescence enhancement could be obtained when doping ratio is 18%.
To reveal the detailed structures of complex NCs, morphologies and size distribution were measured by transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM). As shown in Figs. 2(a) and 2(b), spherical and ellipsoid NCs can be seen clearly. The average size of as-prepared NCs is 2.39±0.267nm (Fig. 2(c)) by statistical analysis, which are similar to other Au-based NCs. In general, Au NCs have cores and regular shape composed of definite number of metal atoms and several satellite motifs.44,45,46 The centralized size distribution suggests that intact NCs have been formed although not uniformly. The formation of various stable NCs is due to the binding of S-Au-S in Au NCs is steadier than that of S-Ag-S in bimetallic AuAg NCs.47 Meanwhile, we conclude that core structures have been slightly changed by Ag doping, because Ag atoms tend to enter the interior of the cores rather than replace Au in the shell units to form relatively more stable structures according to previous theoretical calculations.48 Own to Ag and Au atoms interaction, the 0.231nm and 0.120nm interplanar spacing obtained from our HRTEM photographs is much smaller than the ordinary 0.235nm for Au(111) plane and 0.123nm for Au(311) plane. The overlapped Au and Ag signals in the element mapping pictures also confirm the formation of AuAg NCs (Figs. 2(d)–2(f)).
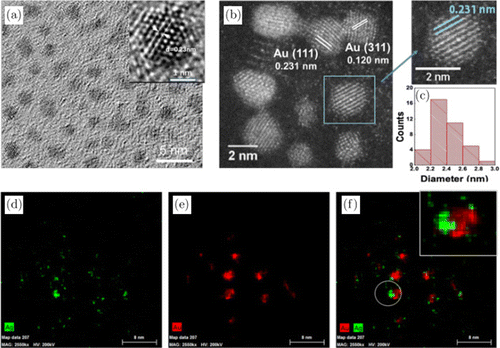
Fig. 2. Morphologic characterization with (a) HRTEM image and (b) TEM image of GSH-protected AuAg NCs with an average size around 2.39nm. The interplanar spacings are 0.231 and 0.120nm, which assign to Au(111) and Au(311) plane. (c) Size distribution of AuAg NCs. Element mapping images of (d) Ag, (e) Au and (f) Ag-Au merged.
To analyze the composition of AuAg NCs, X-ray photoelectron spectroscopy (XPS) and Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI) were performed. In XPS spectra, Au 4f peaks of AuAg NCs are slightly blue-shifted by about 0.3eV compared with the pure Au NCs (Figs. 3(a) and 3(b)) whose binding energies are 83.6eV and 87.3eV. Such phenomenon suggests that Ag doping can induce charge redistribution among Au-Au coupling. In addition, XPS spectra also indicate that Au(III) in chloroauric acid (HAuCl4) precursors have been reduced into Au(0) and Au(I),49,50 and silver atoms have been doped in NCs (Figs. 3(c) and S6). In addition, the relative content of Au:Ag is 15:1 according to XPS analysis. MALDI was further used to analyze the composition of the clusters. As shown in Fig. S7, several peaks can be distinguished in mass spectrum in the range of 4.8–8.6kDa, and could be assigned to a series of clusters denoted by AuxAgy(SG)z, which are mainly composed of Au10Ag1(SG)9, Au11Ag1(SG)12, Au15Ag6(SG)15, Au18Ag4(SG)15 and Au26Ag2(SG)20, respectively.51 Moreover, the average molar ratio of Au:Ag detected by ICP-MS is 9:1, which aligned with MALDI. Figure 3(d) presents the Fourier transformation infrared spectrum (FT-IR) transmission features of GSH ligand and GSH protected AuAg clusters. After reacting with the kernel, several peaks vanished in the spectrum, such as the peak ∼2523cm−1 for S–H stretching and ∼2975cm−1 for –CH3 stretching, suggesting that the S-H bonds of the precursor GSH have been broken and characteristic Au–S or Ag–S bonds have been formed compared with the pure ligand.52 Finally, the AuAg NCs also show similar fingerprint region to other ligand-protected Au series NCs.52,53
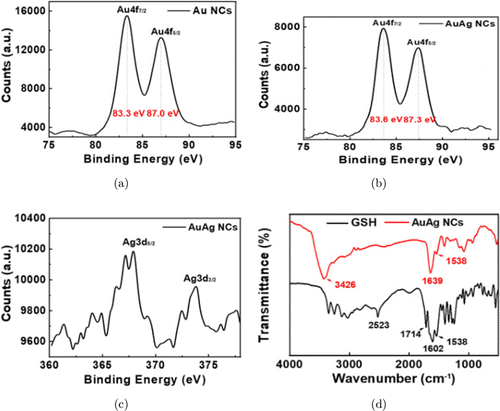
Fig. 3. XPS spectra of (a) Au4f doublet of pure Au NCs and of (b) AuAg NCs, (c) Ag3d doublet of AuAg NCs. (d) FT-IR spectra obtains from pure GSH ligands and AuAg NCs.
The fluorescence lifetime was detected by processing the fluorescence decay curve with biexponential fitting at emission of 605nm. All residuals were fitted with four parameters in two components. Photoluminescence lifetimes are determined by (τ1=3.861μs, k1=50.02%) (τ2=9.601μs, k2=49.98%) for Au NCs and (τ1=4.063μs, k1=50.65%) (τ2=9.424μs, k2=49.35%) for AuAg NCs (Fig. 4). The two weight values in two groups did not show significant differences. The lifetime could be calculated according to the following formula :
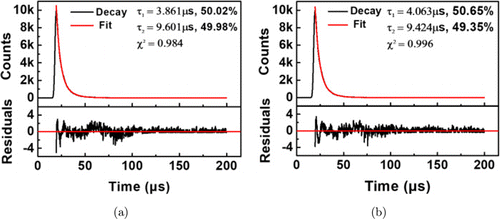
Fig. 4. Photoluminescence lifetime for (a) Au NCs and (b) bimetallic AuAg NCs which are fitted with biexponential curve.
The resultant photoluminescence lifetime of Au NCs and AuAg NCs is 7.594–7.779μs, respectively. The emission lifetime is increased by more than five times compared with the previous AuAg NCs.54,55 Fluorescence lifetime is associated with metal-centered triplet states and charge separated traps.56 Ligand(GSH)-to-metal-metal charge transfer and electron-rich atoms (such as sulfur atoms in GSH) directly donate delocalized electrons to contribute to photoluminescence.12 In AuAg NCs system, both S to Au-Au and S to Au-Ag are possible charge transfer pathways. As discussed above, Ag-ligands could devote more separated charge traps that contribute to long-life fluorescence decay.55,56 In summary, photoluminescence lifetime (∼1 ms) can be increased by Ag doping, and the long lifetime is beneficial to luminescence.
In order to better understand the intrinsic mechanism of the optical properties of AuAg NCs, DFT was used to calculate optical properties using GAUSSIAN57 on supercomputer provided by XSEDE.58 Due to lack of experimentally determined structures, here we constructed two simplified models composed of 1 metal core and 1GSH ligand. The metal core was taken from the well-known Au 13 core with and without silver replacement for the center atom (Au core versus AuAg core), as shown in Fig. 5(a). DFT geometry optimization (B3LYP exchange-correction potential) was used to determine the distance between the core and the ligand. Time-dependent DFT (TDDFT) with exchange-correction potential of M06-2x was carried out to simulate absorption spectra, and the results have been shown in Figs. 5(b) and 5(c). Figure 5(b), left (right), shows the selected density–density respond with iso-surface associated with 349.5nm (Au NCs) and 345.5nm (AuAg NCs). A density–density response is the difference between an excited density of a certain oscillation mode and the ground state density. It is normally used to describe the redistribution of the probabilities of electrons and holes. In Fig. 5(c), a blue-shift is seen clearly in AuAg NCs compared with pure 13-core Au NCs. Note that a faint blue-shift could also be recognized in the experiment (Fig. 1(c)). It implies our theoretical models can provide qualitive explanation to the experiments. Moreover, the density of state (DOS) was employed with Kohn–Sham energies (broadening factor=0.05 atomic unit) which as shown in Fig. 5(d). The comparison of the two systems by aligning the Fermi levels reveals that the enhancement of the absorption in AuAg system may be linked to the increase of DOS above the Fermi level. Thus, the enhancement of fluorescence can be expectable. Due to the limitation of ligand in our model, the ligand to metal ratio was underestimated. Therefore, the underestimation of the enhancement of the spectra and DOS over the experimental results are expected in our simulations.
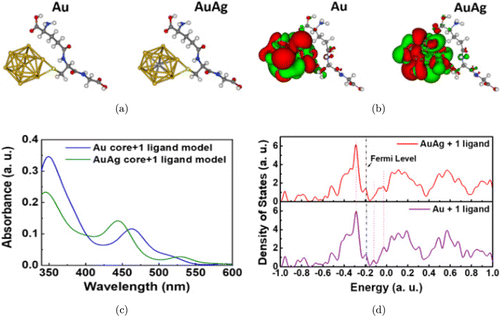
Fig. 5. TDDFT simulation of one metal core connects with single ligand, (a) two simplified model after optimized. (b) Density–density responds for Au (AuAg) model which excited at 349.5nm (345.5nm). Color codes: green for electrons (density excess) and red for holes (density deficiency). (c) The simulation absorption spectra with peaks at 349.5/462.5nm (13 atoms Au core) and 345.5/444nm (12+1 mixed core), a blue-shift appeared and (d) DOS analyzed by Kohn-Sham energy with broadening factor of 0.05 atomic unit.
The in vitro or in vivo toxicity of NCs are critical to biomedical applications. The cytotoxicity was investigated as shown in Fig. S8. Chinese hamster ovary (CHO) cells were cultured with different concentration AuAg NCs of 0.1∼3.2mM for 24 and 48 h. The MMT assays show excellent biocompatibility that AuAg clusters cannot cause significant effects on cellar viability up to 3.2 mM in 24 h and 0.2 mM in 48 h. Thus, we employed high dosages of AuAg NCs in toxicological experiment. C57 mice were tested with injection of 0.2 mL (2 mg/mL) AuAg NCs by intravenous injection. As shown in Fig. 6(a), there are no significant differences in weight between two groups after 1 day and 30 days treatment, which indicates negligible adverse effect on mice. It can also be seen that most of NCs were fast excreted by bladder with obvious signal during in vivo fluorescence imaging (Fig. 6(b)) at a dose of 400μg by intravenous injection. Then, we selected the following hematology and biochemistry parameters for evaluation and analysis, including white blood cell (WBC), red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and blood platelet (PLT). Figure 6(c) exhibits that almost every indicators of the treated mice remain the same as the control group, which indicates that the NCs have limited harm to mice. To complete research on the toxicity of NCs in vivo, we performed a battery of biochemistry in a period of 30 days, including alanine transaminase (ALT), aspartate transaminase (AST), total protein (TP), albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), globulin (GLOB) and total bilirubin (TBIL). As shown in Fig. 6(d), some indicators behaved abnormal in the first day after injected, including ALT, AST, BUN and CREA. These indicators mainly reflect liver and kidney functions and activities. Nevertheless, all of them returned to normal after 30 days, which indicates that NCs could cause slight damage or response, but these limited inflammations could self-recovered by immune system of mice. The results that in vivo toxicities could be ignored in certain reasonable range of concentration. Meanwhile, the toxicity results were also checked up with the excretion experiments where NCs could be quickly cleared by kidney with ultrasmall size below 5.5nm.59 Excellent biocompatibility is an indispensable prerequisite for the following in vivo fluorescence imaging.59 Similarly, with the low toxicitiy, Au NCs have been widely reported on their predominant effect on radiation therapy and metabolic diseases.60,61
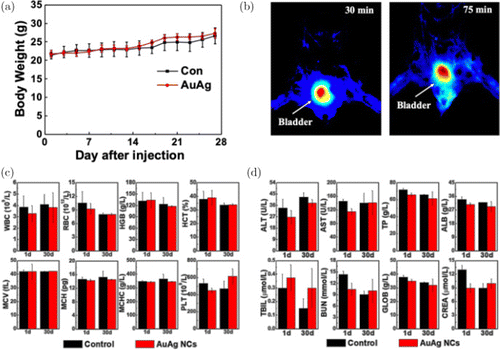
Fig. 6. In vivo toxicity detections, including (a) changing trend of mice’s weight in the following 4 weeks after injection, (b) biodistribution of 30min p.i. and 75min p.i. by in vivo fluorescence imaging and (c–d) Mice’s hematology data and blood chemistry which were acquired at 2 time points (1d/30d). Mice were treated with 0.2mL (2mg/mL) AuAg NCs via administered intraperitoneal injection once at first day. Data were counted by Student’s t-test.
Encouraged by the high QY of AuAg NCs, we then investigated in vivo bioimaging. First, local tissue imaging was performed using two-photon excitation fluorescence microscopy (2PM). As an advanced imaging method, two-photon imaging could attain deep penetration with the lowest phototoxicity owning to its long-wavelength near-infrared excitation, which has great potential in real-time in vivo imaging and clinical diagnoses. In the past few years, graphene quantum dots have been proposed by two-photon excitation and biocompatible.62 Meanwhile, as mentioned above, low toxic and high QY AuAg NCs are also competitive candidates for fluorescence probes. 2PM images have been shown in Fig. 7, balb/c mice were injected 200μl (18mg/mL) AuAg NCs by intravenous injection, the cerebral vessels were observed clearly under the excitation of 800nm. Figures 7(a) and 7(b) display the mice’s brain vessels “lightening” homogeneously by AuAg NCs whose diameter and morphology are sharp and clear. Meanwhile, these clusters also owned marvelous stability and persistent brightness, as shown in Figs. 7(c)–7(f), which is consistent with the florescence stability analysis (Fig. S5).
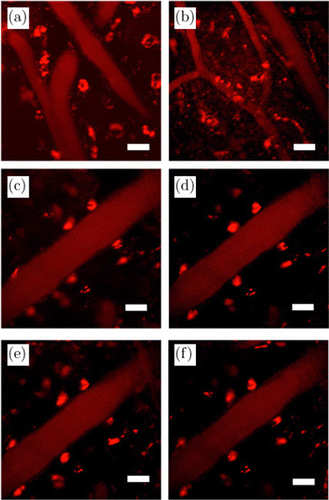
Fig. 7. 2PM images of balb/c mice’s brain vessels with (a–b) different visual fields, taken with 800nm two-photon excitation, the scale bar stands for 20μm. (c–f) Focus on single brain vessel at varied time points of 1/5/10/15min after post injection (p. i.).
Our studies are based on previous studies63,64 and made an approach to enhance their luminescence. To date, the multifunctional Au or Ag NCs have been deeply explored, the great potential of application in biomedicine has been discovered, which is not only in bioimaging, but also in the field of healthy protection and catalysis.65,66,67,68 Nevertheless, further research on clusters including improving fluorescence lifetime and extending the penetration depth in the tissue over extended periods of time need to be performed.
4. Conclusions
In conclusion, we successfully synthesized AuAg NCs by simplified one-pot route at different silver doping levels. A series of characterizations is investigated to analyze and evaluate the effects of Ag doping on alloy clusters. AuAg NCs exhibit ultrasmall size (∼ 2.4 nm) and ultrabright of more than 7.5-fold enhancement in fluorescence compared with pure Au NCs. Furthermore, AuAg NCs also exhibit high stability and long fluorescence lifetime, which are critical for potential biological applications. Moreover, the significant differences between Au NPs and Au NCs were discussed. DFT calculations suggest that the enhancement of the QY and the fluorescence are due to extra states above the Fermi level induced by sliver doping. In addition, AuAg NCs have limited toxicities on mice according to our in vitro and in vivo tests. Inspired by their incredible biocompatibility we further employed them as biological probes in 2PM imaging to realize mice’s brain vessel real-time imaging. Strong and high-resolution signals can be obtained, illustrating that AuAg NCs could become a new candidate of fluorescence probe for in vivo imaging.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 11804248, 91859101, 81971744, U1932107), the Foundation of Tianjin University and Natural Science Foundation of Tianjin (Grant No. 18JCQNJC03200). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1548562.
Conflict of Interest
The authors declared that they have no conflicts of interest to this work.


