Tumor cell death visualization of renal cell carcinoma under the combined effect of the Gratiola officinalis extract and cyclophosphamide using fluorescent staining methods
Abstract
Objective of the study: We used fluorescence imaging methods of apoptosis and necrosis in human renal carcinoma A498 tumor cells in vitro to reveal the indicated forms of cell death under the combined effect of flavonoid-containing extract of Gratiola officinalis and cytostatic (cyclophosphamide). Materials and methods: The dyes were propidium iodide and acridine orange, which were used in the “alive and dead” test. This test helped us to identify the total number of dead cells in the forms of necrosis and apoptosis and the number of cells in which apoptosis had started, it was characterized by the appearance of apoptotic bodies or nucleus pyknosis. Results: We found the most pronounced cytotoxic activity at the ratio of extract of Gratiola officinalis and cyclophosphamide concentrations of 1:1. The number of living cells decreased when exposed to the ratio of extract and cytostatic concentrations of 2:1. When the ratio of concentration of the extract relative to the cytostatic increased to 3:1, the cytostatic activity of the extract began to appear, the total number of tumor cells decreased. The number of cells with nucleus pyknosis and the number of cells with apoptosis signs significantly increased at a 3:1 ratio of extract and cytostatic concentrations, which confirms the presence of pro-apoptotic activity of the studied combination. This trend indicates the dependence of a certain form of cell death (apoptosis, necrosis) on the ratio of extract and cytostatic doses, and it also demonstrates the cytostatic and cytotoxic effects of this combination. Conclusion: Fluorescence methods of investigation in the “alive and dead” test allowed us to visualize the forms of cell death of human kidney carcinoma A498 by combined exposure to the flavonoid-containing extract of Gratiola officinalis and cytostatic (cyclophosphamide) 24 h after exposure. We found that the combination with a concentration ratio of the extract and cyclophosphamide of 3:1 has the greatest effectiveness due to stimulation of the cytostatic effect and cytotoxic effect.
1. Introduction
A large number of methods for studying cell death exist nowadays; these methods allow to determine genetically programmed death (apoptosis, autophagy) and unprogrammed death (necrosis).1 These observation methods are based on recording the morphological, biochemical and molecular changes in cells.1,2 However, fluorescence microscopy is one of the most informative methods of investigation. It is relevant in the study of phases of tumor cell life cycle affected by antitumor drugs. Fluorescence microscopy is used to evaluate the effectiveness of these drugs, and it is also used to detect tumor resistance to therapy. The object of study is a culture of tumor cells, the cells can present three variants of life cycle termination: apoptosis, necrosis and autophagy.3 The method of fluorescent microscopy with fluorescent dye is used to detect these cell types. Acridine orange is a dye that specifically binds to DNA.4,5 Vitally stained cells are examined both in suspension and as fixed.
A sign of apoptosis using fluorescence microscopy is the detection of brightly glowing condensed chromatin. Propidium iodide is used for quantitative cell counting.6,7 The combination of acridine orange and propidium iodide presents a “live and dead” test; it makes it possible to detect the total number of dead cells, as well as to differentiate them according to the form of death. At the same time, cells subjected to necrosis are stained red, while the cells with developed apoptosis are stained green. In the cells where apoptosis has begun, apoptotic corpuscles or pyknosis of the nucleus are detected.
Several factors in experiments in vitro must be considered when interpreting the results obtained: lack of microenvironment, disruption of intercellular contacts, formation of tumor vascularization and lack of barrier function of the immune system. These factors create incomplete correspondence of tumor susceptibility conditions to chemopreparations, relative to in-vivo experiments.8 The ability to interact with each other is one of the features of anticancer drugs. This ability initiates the development of synergistic, additive and antagonistic effects.9 However, study of drugs on tumor cell cultures helps to establish the direct cytotoxic effect of a chemo-drug or its combination by quantitative and qualitative assessments of cancer cell death and proliferative activity. Research using tumor cell culture allows monitoring of chemosensitivity and investigating the mechanisms of general drug and cross-resistance of neoplasia during their progression and at different stages of chemotherapy in patients.10In-vitro study models allow screening of potential and new antitumor agents in order to predict their effectiveness and exclude ineffective compounds.10
Currently, a relevant area of research is the evaluation of combined therapy of tumor process, where the therapy includes simultaneous use of flavonoid-containing extract and classical chemotherapeutic drug.
Flavonoid-containing extract of Gratiola officinalis is obtained by a method that reduces toxicity and increases the yield of flavonoids from plant raw materials, it has antitumor, anticancer and immunomodulatory effects. The method of obtaining a dry extract of herbal raw materials includes grinding the herb Gratiola officinalis, extraction with 96% alcohol, evaporation of the extract, addition of chloroform and removal of chloroform. Extraction with alcohol is carried out in a water bath until boiling and boiled for 14–15 min, evaporated at a temperature of 55–60∘C, the evaporated extract is first diluted with distilled water at 40–50∘C, then chloroform is added in the proportion 4/5 of water and 1/5 of chloroform, cooled to room temperature and centrifuged at a speed of 1500 rpm for 15min, then the water fraction is separated and dried.11 The results of studies allowed us to describe the mechanisms of action on the tumor and tumor cells: cell cycle arrest at G0-phase, triggering of apoptosis induced by p53-gene and suppression of cytoprotective autophagy.12,13
Cyclophosphamide is used as a classic chemotherapeutic drug. It has cytostatic and immunosuppressive effects. Antitumor action is realized directly in tumor cells, where cyclophosphamide is biotransformed under the action of phosphatases with the formation of an active metabolite with alkylating effect.
There are known methods for combining cytostatic (cyclophosphamide) with flavonoid-containing extracts of common mountain ash, black chokeberry and Rhodiola rosea. However, the data described in the literature refer to the experiments in vivo; but the number of in-vitro studies on cell cultures is very limited.14,15,16
Objective of the study. We used fluorescence imaging methods of apoptosis and necrosis in human renal carcinoma A498 tumor cells in vitro to reveal the indicated forms of cell death under the combined effect of flavonoid-containing extract of Gratiola officinalis and cytostatic (cyclophosphamide).
2. Materials and Methods
The study object was renal carcinoma cells A498, they were obtained from the Bank of Cell Cultures of the Russian Cancer Research Center named after N.N. Blokhin, Moscow. The cells were cultured in plastic vials in RPMI 4 medium (10% fetal serum, ampicillin, sodium pyruvate and glutamine) in a CO2 incubator at 37∘C for 24h. Then the cells in plastic vials were randomly divided into groups: control group (no exposure) and experimental groups (groups with exposure to Gratiola officinalis extract, cyclophosphamide and Gratiola officinalis extract in combination with cyclophosphamide in the ratios of 1:1; 1:2; 2:1 and 3:1). Concentrations of the studied substances: Gratiola officinalis extract: 100μg/mL, cyclophosphamide: 300μg/mL. These concentrations were used from the calculation of the minimum effective concentrations for each of the studied substances, the calculation was made experimentally. We studied three variants of the ratios of Gratiola officinalis and cyclophosphamide concentrations (1:1; 1:2; and 2:1, 3:1), the comparison was made with the control group (without substance exposure). These ratios were chosen based on pilot experiments in which they were most effective. The cells were then cultured for 24 h, then they were stained. We used the following as dyes: propidium iodide, which penetrates nonviable cells by destroying their membrane, and acridine orange for staining live cells.
We used a Nikon microscope (Tokyo, Japan) to visualize cells. Images were captured and analyzed using a Nikon digital video camera (2560 × 1920 resolution). Fiji Image J and NIS-Elements BR 4.60 software were used for cell counting. To analyze cytotoxic, cytostatic and apoptotic activities, we compared the following parameters in the control and experimental groups: total number of cells per field of view, number of dead cells per field of view, number of live cells per field of view and number of cells with signs of apoptosis (apoptotic cells, nucleus pyknosis, “sickle” chromatin). Statistical processing was performed using SPSS Statistics 17.0 program. Normality of feature distribution among the groups was assessed using Shapiro–Wilk criterion. The distribution of the signs did not correspond to normal distribution, so we used nonparametric Mann–Whitney U-test to assess the reliability of differences from the control group. Median, first and third quartiles as well as maximum and minimum values were determined in the groups. Differences were considered significant at the significance level of p < 0.05.
3. Results
Fluorescence microscopy 24h after the experimental exposure revealed a halving of the total number of cells when exposed to the Gratiola officinalis. In addition, there was a 61% decrease in the number of live cells relative to the control group (Table 1).
The study of cells after exposure to cyclophosphamide showed a significant increase in the number of dead cells, it was four times higher than in the control group (Table 1).
The control group was dominated by cells in a state of preparation for apoptosis (with sickle-shaped nuclei) and cells in a state of mitosis [Fig. 1(a) white arrows and Fig. 1(b)], while in the group of combined exposure to the Gratiola officinalis in a 1:1 ratio most cells have already died, which resulted in a decrease of the total cell number and as a result a 4.5-fold decrease of live cells in the cell population. However, there was an increase in the number of cells with apoptotic corpuscles, which indicated the activation of apoptosis in this group [Fig. 1(c) white arrow and Fig. 1(d)].
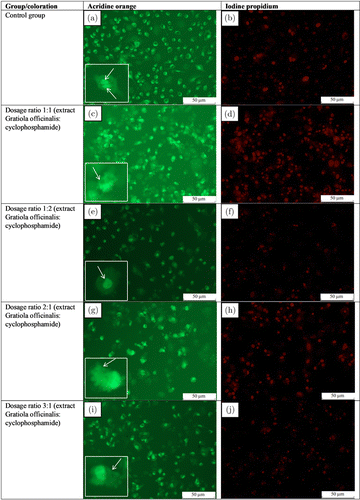
Fig. 1. Culture cells A498 at 24h after exposure to the combination of Gratiola officinalis extract and cyclophosphamide in the ratios of 1:1, 1:2, 2:1 and 3:1: Panels (a), (c), (e), (g) and (i) — coloring of dead cells with acridine orange chromatin nuclei of living cells; panels (b), (d), (f), (h) and (j) — coloring of dead cells with iodine propionate. Objective: 20×.
A twofold increase in the concentration of cyclophosphamide in relation to the Gratiola officinalis extract (1 concentration of extract:2 concentrations of cyclophosphamide) resulted in a 39% decrease in the number of living cells relative to the control group, but this was not due to an increase in dead cells or those entering the apoptosis path, the cause was the increased cytostatic activity of this combination, which led to an inhibition of cell division and a decrease in the total cell number. Cytostatic activity was also confirmed by the absence of mitosis in the cells [Figs. 1(e) and 1(f)].
A twofold increase in the concentration of the Gratiola officinalis extract relative to cyclophosphamide (2 concentrations of extract:1 concentration of cyclophosphamide:) also showed a 72% decrease in the number of living cells relative to the control group, both by reducing their total number and by increasing the number of dead cells. An increase in apoptotic activity was observed, as evidenced by an increase in the number of cells with apoptotic corpuscles [Fig. 1(g) white arrow and Fig. 1(h)].
When the cell culture was exposed to a 3:1 ratio of extract and cytostatic concentrations, a twofold decrease in the total number of cells and the number of living cells was observed, while there was no increase in dead cells, but the number of cells with nucleus pyknosis and apoptotic teles was significantly increased, which is a predictor of triggering apoptosis mechanisms [Fig. 1(i) white arrow and Fig. 1(j)].
The number of kidney cancer cells with sickle-shaped nuclei was reduced in all experimental groups from 73% to 94%. However, the number of cells with nucleus pyknosis and apoptotic corpuscles, indicative of cell preparation for apoptosis, was significantly higher than control values only at the 3:1 concentration ratio of Gratiola officinalis and cyclophosphamide (Table 1).
| Group | Control Median(Q1–Q3)[min–max] | 1:1 Median (Q1–Q3)[min–max] | 1:2 Median (Q1–Q3)[min–max] | 2:1 Median (Q1–Q3)[min–max] | 3:1 Median (Q1–Q3)[min–max] | Extract Median(Q1–Q3)[min–max] | Cyclophosphamide Median(Q1–Q3)[min–max] |
|---|---|---|---|---|---|---|---|
| Number of cells in the field of vision (NC) | 214(157–236)[106–279] | 189(109–215)[54–252] | 163(83–193)[34–248] | 131* (66– 137)[24– 195] | 123* (103– 161)[52– 202] | 122* (89– 144)[60– 193] | 201(185–224)[164–246] |
| Number of dead cells in the field of vision | 40(34–56)[14–75] | 140* (62– 76)[28– 212] | 62(27–76)[17–103] | 55(46–89)[52–104] | 50(26–71)[22–97] | 57(45–67)[34–86] | 159* (150– 181)[164– 205] |
| Number of live cells in the field of vision | 167(125–180)[85–223] | 38* (25– 47)[0– 76] | 101* (56– 117)[17– 150] | 48* (30– 82)[16– 91] | 85* (72– 95)[26– 107] | 65* (32– 87)[23– 98] | 42* (26– 45)[13– 67] |
| Number of cells with sickles | 15(11–24)[9–33] | 2* (0– 3)[0– 17] | 1* (0– 4)[0– 16] | 4* (1– 9)[1– 12] | 3* (2– 5)[1– 10] | 2* (1– 3)[0– 7] | 3* (2– 7)[0– 11] |
| Number of cells with nucleus pyknosis | 2(2–7)[1–11] | 4(2–5)[0–8] | 3(2–10)[1–17] | 7(4–9)[1–13] | 17* (10– 24)[1– 27] | 7(5–12)[0–19] | 5(2–9)[1–16] |
| Number of apoptotic cells | 3(2–5)[1–12] | 4(3–9)[0–12] | 2(2–3)[1–6] | 7(3–9)[2–17] | 6* (5– 11)[3– 13] | 6(4–11)[1–16] | 5(3–15)[1–19] |
4. Discussion
As a result of the analysis of the obtained results on the study of the forms of tumor cell death of kidney cancer A498, by fluorescent methods of imaging cell death by staining with propidium iodide and acridine orange, the most effective ratio of concentrations of the Gratiola officinalis extract and cyclophosphamide was found.
The most pronounced cytotoxic activity was exhibited by the ratio of concentrations of the extract and cyclophosphamide of 1:1. Similar data were obtained when analyzing the number of dead cells in the group with isolated exposure to cyclophosphamide, which indicates an insufficient concentration of the Gratiola officinalis extract in the ratio of 1:1.
In all groups under study, there was a decrease in the number of living cells, indicating cytostatic effects on tumor cells, slowing the processes of division and proliferation, except for the control group, where the figures of mitosis were observed. The number of cells with nucleus pyknosis and the number of cells with signs of apoptosis increased significantly at a 3:1 ratio of extract and cytostatic concentrations, which confirms the presence of pro-apoptotic activity of the studied combination. This trend indicates the dependence of a certain form of cell death (apoptosis, necrosis) on the ratio of doses of the extract and cytostatic, and it also demonstrates the cytostatic and cytotoxic effects of this combination.
Given that cyclophosphamide belongs to the class of alkylating compounds, the effect of which is primarily due to modification of purine and pyrimidine bases, as a result of this the integrity of DNA molecules is violated. Cells after exposure to alkylating compounds stop in the G1-phase and do not enter the S-phase. Tumor tissue cells are highly sensitive to these substances. Its properties are related to the induction of single-strand breaks in DNA and the formation of cross-links between DNA molecules.17
At the cellular level, the mutagenicity of cyclophosphamide is tested by the occurrence of a large number of exchanges between sister chromatids and the appearance of cells with numerous micronuclei. When cyclophosphamide at a concentration of 300μg/mL is applied to cultured cells individually, the mitotic index increases slightly due to an increase in the number of cells with pathological mitoses, which caused no difference in the total number of cells between the group with isolated exposure to cyclophosphamide and the control group.17,18
The action of cyclophosphamide leads to cell death in interphase, to the appearance of cells with signs of “mitotic catastrophe” and causes structural and functional imbalances of the genome in some cells that have completed division. At the same time, the effect of cytostatic action of cyclophosphamide on cultured cells depends on the dose of the drug and the time of its exposure.19
In previous studies, the effect of Gratiola officinalis extract in ultra-low concentrations on the activation of apoptosis in tumor cells has been proven.20,21 In this study we observed a tendency in modulation of cytostatic and cytotoxic effects of cyclophosphamide and simultaneous activation of apoptosis processes, significantly reducing the population of tumor cells.
5. Conclusion
Thus, the use of fluorescent methods in the “alive and dead” test allowed to visualize cells with different forms of cell death (apoptosis, necrosis) of human renal carcinoma A498 under the combined effect of flavonoid-containing extract of Gratiola officinalis and cytostatic (cyclophosphamide) at 24 h after exposure. It was found that the combination with a concentration ratio of the extract and cyclophosphamide of 3:1 has the greatest effectiveness due to the modulation of cytostatic and cytotoxic effects.
Conflict of Interest
The authors declare that there are no conflicts of interest relevant to this paper.
Acknowledgments
The work was supported by State Assignment No. 121032600197-2 of the Health Ministry of Russia.


