A pilot study of the dynamics of tissue oxygenation in vivo using time-resolved phosphorescence imaging
Abstract
Oxygenation of tissues plays an important role in the development and progression of tumor to treatment effects. Method of metalloporphyrines phosphorescence quenching by oxygen is one of the ways to measure dynamics of the oxygen concentration in the tissues by phosphorescence lifetime imaging of meso-tetra(sulfopheny1)tetrabenzoporphyrin Pd (II) (TBP) using the time-correlated single photon counting (TCSPC) method. It has been shown that phosphorescence lifetime of the sensor in S37 tumor in vivo varied in the range of 130 to 290 μs after both topical and intravenous administration of TBP. It indicates that oxygen level in tumors was lower compared to normal tissues where TBP phosphorescence has not been detected. Phosphorescence lifetimes of TBP increased in the solid tumor and in the muscle after photodynamic therapy of solid tumor that demonstrates oxygen consumption during treatment and possibly stopping the blood flow and hence the oxygen supply to the tissues.
1. Introduction
Hypoxia or low oxygen concentration in tumor is an important factor of its growth and sensitivity to different anticancer drugs.1,2 Oxygen in tumors has nonuniform distribution.3 The latter circumstance is connected, apparently, with the morphological features of the circulatory network of the tumor. Tumor blood vessels are known to be atypical.4 Most often, they represent the sinusoidal type vessels with thin walls and wide lumen. The wall of vessels in tumor is often represented by a single layer of endothelial cells located directly on tumor tissue or formed by the cells of the tumor itself (open blood circulation system in the tumor), and the structure of the circulatory network will vary from one tumor to another. All these features lead to the fact that the distribution of oxygen will be different in two different samples of tumors of the same type5 and can strongly influence efficiency of tumor treatment especially photodynamic therapy (PDT).6 Therefore, it is important to study the intratumoral distribution of oxygen in each case. Hypoxia also plays an essential role in the wound repair.7,8
There are a number of methods for the detection of oxygen in the tissue. They can be divided into direct and indirect, invasive and noninvasive methods. Invasive polarographic methods are the most popular in determination of oxygen status of the tissues.9 Recently, several promising less-invasive methods for the evaluation of hypoxia were developed, but their widespread introduction into the clinical characterization of the tumor is difficult due to various reasons, including uncertain correlation between the hypoxia and potential treatment of the tumor, i.e., currently available knowledge do not allow to say whether the cells with a specific oxygen status will be sensitive or insensitive to the intended treatment.
In addition to the fact that the use of invasive methods for the determination of oxygen in tumors is not desirable, invasive methods allow to determine the oxygen content of only one selected point. In experimental oncology, the most popular method is the luminescence imaging using various luminescent probes.10
Oxygen sensors are usually based on luminescence quenching phenomenon.11 To exclude influence of the concentration variations in case of intensity-based measurements it was proposed to use sensors, which have oxygen-dependent phosphorescence and oxygen-independent fluorescence.12
Most commonly used are sensors based on luminescence quenching by molecular oxygen that include trypaflavine, eosin, erythrosine, polycyclic hydrocarbons, polypyridine complexes of transition metals, cyclometallated complexes, complexes with ruthenium ions and metalloporphyrin’s.8,13,14,15,16
The presence of a heavy atom in luminescent probe as a central ion or substituent in the ligand usually significantly increases the probability of the intercombinational conversion to the triplet state that increases the phosphorescence efficiency.
In the presence of molecular oxygen luminescence of the molecules is quenched by radiationless deactivation during the molecular interaction between the quencher and the luminophore (dynamic quenching), which is limited by the diffusion rate. One of the generally accepted mechanisms assumes that oxygen initiates intercombinational conversion of the luminophore from triplet state to the ground state, while the molecular oxygen goes into the excited state (1Δg, the first excited state or 1Σg+, second excited state) and then returns to the ground state (3Σg−, triplet state). Direct evidence of such mechanism of energy transfer is the formation of singlet oxygen (1O2). However, quenching may also be affected through transfer of electrons, for example, in case of cyclometallated iridium complexes.17
One of the ways to determine the concentration of oxygen in the tissue is the measurement of sensor’s luminescence lifetime (τ), which has two main advantages compared to the measurement of the luminescence intensity. First, the luminescence lifetime does not depend on the incident intensity of light, allowing use of various concentrations of sensor. Second, this approach has relatively high sensitivity due to the low values of background fluorescence.8,10
In this study, we propose to determine the dynamics of tumors oxygen status by measuring sodium salt of meso-tetra(sulfopheny1)tetrabenzoporphyrin Pd (II) (TBP, Fig. 1) phosphorescence using the approach described elsewhere.18
Fig. 1. Sodium salt of meso-tetra(sulfopheny1)tetrabenzoporphyrin Pd (II) (TBP).
2. Materials and Methods
2.1. Equipment for the phosphorescence lifetime measurement using phosphorescent imaging method
All measurements were performed on a scanning laser system DCS-120 MACRO (Becker&Hickl GmbH).19,20 A pulsed laser with 40MHz repetition rate was used (Fianium, SC-400, UK) for the excitation. The signal was detected using HPM-100-40 detector (Becker&Hickl GmbH) with a sensitivity ran ge from 400 to 900nm. Measurements were performed at 640 nm excitation using 780nm long pass and 845nm band pass filters (780LP and HQ845/55 respectively, Chroma, US). Phosphorescence images were processed using the SPCImage software (Becker& Hickl GmbH, Germany). The decay curves were fitted using mono-exponential model.
2.2. Animals and tumors
We used CBF1 mice (CBAxC57Bl/6j), females, 20–22g weight, from the N.N. Blokhin RTSC of the RAMS nursery, and outbred white mice, males, from “Stolbovaya” brider. All animal studies were conducted in accordance with national requirements for the human treatment of experimental animals. Throughout the experiment mice received certified complete feed “Chara” (free from background fluorescence, “Assortiment Agro”, Russia) and water without restrictions.
Murine sarcoma S37 was used as a model of tumor growth.21 The tumor was inoculated to CBF1 mice subcutaneously into the external side of the calf muscles of one or both hind legs with 0.05 mL of ascitic fluid diluted to 4 times with sterile 0.9% sodium chloride solution. Day of inoculation was considered as zero day of tumor growth.
2.3. Study of the TBP phosphorescence lifetime in animal tissues
The study was conducted on 6–11th days of tumor growth. TBP (provided by Prof. E. A. Lukjanetz, Organic Intermediates & Dyes Institute, NIOPIK, Moscow) was injected in 0.9% sodium chloride solution intratumorally or systemically (intravenous). At intratumoral injection (Table 1), TBP was used at the concentration of 2mg/mL in a volume of 20μL. One injection of TBP was given to the tumor, the second into the muscle. Then, phosphorescence lifetime was measured at 5, 30 and 50min after injection. At systemic (intravenous) injection (Table 2), TBP was injected in the dose of 20 mg/kg into the lateral tail vein. The phosphorescence was detected in the tumor in vivo at different times after the injection of TBP from 1 to 24h.
| An average phosphorescent lifetime τph±σ (μs) | |||||
|---|---|---|---|---|---|
| Time after injection (min) | |||||
| Sample (mouse) | Day of growth | 5 | 30 | 50 | Postmortem |
| 1 | 6 | 270 ± 30 | 280 ± 20 | 290 ± 20 | 310 ± 20 |
| 2 | 10 | 280 ± 90 | 280 ± 30 | 260 ± 30 | n/d |
| 3 | 11 | 230 ± 30 | 240 ± 40 | 250 ± 30 | n/d |
| 4 | 6 | 170 ± 20 | 220 ± 20 | 230 ± 20 | 300 ± 10 |
| An average phosphorescent lifetime τph±σ (μs) | |||||||
|---|---|---|---|---|---|---|---|
| Time after injection (min) | |||||||
| Sample (mouse) | Day of growth | 10 | 30 | 60 | 120 | 240 | 1440 |
| 5 | 6 | 230 ± 70 | 280 ± 90 | 240 ± 40 | 230 ± 30 | 190 ± 30 | 220 ± 30 |
| 6 | 10 | 190 ± 70 | 130 ± 20 | 130 ± 20 | 130 ± 20 | 170 ± 20 | n/d |
| 7 | 11 | 260 ± 70 | 280 ± 70 | 220 ± 30 | n/d | ||
After in vivo measurements mice were sacrificed by cervical vertebrae dislocation under anesthesia, and then within few minutes another measurement of the tumor phosphorescence lifetime was carried out when temperature of the mouse body practically do not change. The day before the start of the experiment, the tumor growth area was epilated. Right before starting the tumor phosphorescence lifetime measurement mice were anesthetized by intramuscular injection of 1.25mg/kg of tiletamine hydrochloride, 1.25mg/kg zolazepam hydrochloride and 10 mg/kg xylazine hydrochloride.
2.4. Study of the influence of PDT on the phosphorescence of TBP
PDT was performed in mice grafted with tumors onto both paws on the 7th day of their growth. One of the experimental tumors were further subjected to PDT (treated tumor), the other was left intact in relation to light exposure (control tumor).
24h before PDT, mice were injected with aluminum sulfophthalocyanine (drug “Photosens”, Organic Intermediates & Dyes Institute, NIOPIK, Moscow) intravenously at a dose of 5 mg/kg. 1 h before PDT, TBP in a physiological solution was injected intravenously at a dose of 20mg/kg. 40 min after the injection of TBP tumor phosphorescence lifetime was measured. Further, PDT (λ=680nm, the energy density of 130J/cm2, power density of 150mW/cm2) of one of the tumors during 15min has been performed. After PDT phosphorescence lifetimes of the experimental and control tumors were measured.
The antitumor effect was evaluated by the ratio of the size of experimental and control tumors: T/C where T and C are the volumes of the experimental and control tumors, respectively. The volume of a tumor node was determined by the following formula (Eq. (1)):
In the tables and text, M ± m values are given.
3. Results and Discussion
3.1. Phosphorescent properties of TBP
According to our experimental results, TBP is not phosphorescent in buffered solution at pH 7.5 or in the same buffered solution contained 2% BSA saturated with oxygen (22kPa) (Fig. 2(c)). The phosphorescence lifetime of TBP in the same deoxygenated solutions was about 105 and 305μs in the presence of 2% BSA (Figs. 2(b) and 2(d)).
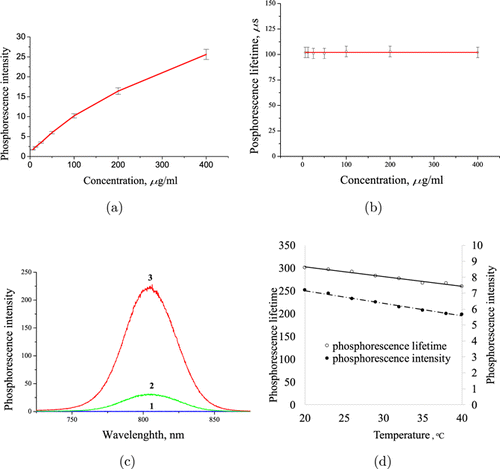
Fig. 2. Phosphorescent properties of TBP in buffered solution: Na2SO3 — 40mg/mL, NaH2PO4 — 20mg/mL, pH 7.6. (a) Concentration dependence; (b) phosphorescent lifetime at different concentrations; (c) spectra: 1 — oxygenated solution (without Na2SO3), 2 — deoxygenated solution (plus Na2SO3), 3 — deoxygenated solution plus 2% BSA; (d) phosphorescent lifetime and intensity dependence on temperature. Cary Eclipse fluorometer, excitation 630nm, slits −10nm, delay line 100μs.
Phosphorescent intensity of TBP is dependent on concentrations may be due to some degree of aggregation or self-quenching at high concentration (Fig. 2(a)). Because both mentioned mechanisms kinetically described as static type of quenching, the phosphorescence lifetime is independent on probe concentration (Fig. 2(b)). Binding of TBP to certain proteins, for example to bovine serum albumin, can lead to disaggregation and minimize phosphorescence quenching by water (Fig. 2(c)). Temperature variation in the physiological range of 36–38∘C significantly influence less the phosphorescent signal variation (Fig. 2(d)).
3.2. Experimental tumors and TBP luminescence in mice tissues
The tumor sarcoma S37 is maintained in ascitic form, while solid form is used for the experiments. It is noted that behavior of this tumor is dependent on the type of mice. So, grafting this tumor to the outbred mice results in tumor resorption in 70% of cases, and only in 10% of cases while using the BalbC mice. In the present work, we used a solid variant of sarcoma S37, grafted to mice hybrids CBAxC57Bl/6j. Cases of spontaneous tumor resorption were not fixed neither in our experiments nor reported in literature. Grafted tumors represented either nodular or diffuse formations. In the first case, the tumor could be palpated in vivo. Upon dissection the blood vessels were visible on its edge. The tumor center was white with a yellowish tinge on the early stages of tumor growth (7–11 days of growth), then it was necrotic with the formation of necrotic scab at the site of growth of the tumor to the skin. Later, the marginal growth of the tumor was observed.
Diffuse tumors were located directly on the muscle, their color was pale pink for observation periods up to 14th day inclusively, that is caused, apparently, by the presence of blood capillaries in the tumor. Macroscopic areas of necrosis in these tumors were not detected until the 14th day of growth.
Both macroscopic types of the tumor were used in the study of the luminescence properties of TBP. In the study of the phosphorescence of TBP in mice tumors without additional treatment dye was injected intratumorally and systemically intravenously.
In Ref. 22, another value of lifetime of the TBP-based oxygen sensor, 600–800 μs, is described. This value of lifetime is missing in vitro and appears in vivo (Fig. 3) in mice treated with tetrapyrrole compounds — TBP or aluminum sulfophthalocyanine (drug “Photosens”) and apparently caused by the later compound. Further, in this study the phosphorescence lifetime of the tissues of mice treated with TBP was calculated in the range from 0 to 400μs.
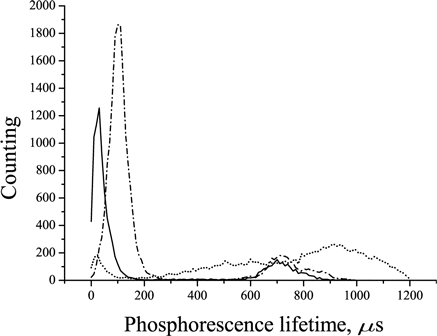
Fig. 3. Phosphorescence lifetime distribution in the tumors of control mice without any impacts (solid line), animals treated only with “Photosens” (dashed line) and treated with TBP intravenously at a dose of 25 mg/kg (point and line).
The phosphorescence lifetime of the S37 tumors in mice without the oxygen sensor was 11± 1μs that is more than an order of magnitude lower than that of the dye and therefore should not make a significant contribution to the determination of the phosphorescence lifetime of TBP.
Under topical application, the phosphorescence of the dye was determined when used in concentrations of 0.2 and 2.0mg/kg. At a concentration of 0.02mg/mL TBP phosphorescence was not detected.
In vivo the average phosphorescence lifetime of TBP in the tumor ranges from 169 to 292μs in different tumors, which probably depends on the site of injection of sensor into the tumor (and the oxygen content there). In muscle, TBP phosphorescence was not detected except for the site of the dye injection, where ischemia occurred in response to mechanical tissue damage as a protective mechanism to prevent bleeding (Fig. 4).
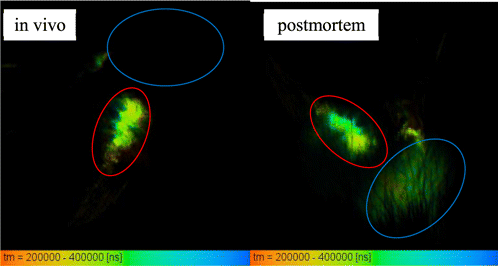
Fig. 4. Time-resolved phosphorescence images of S37 tumors of mice with intratumoral (red ROI) and intramuscular (blue ROI) application of 20μL of 20mg/mL TBP, left — in vivo, right — after sacrificing.
When administered intravenously, TBP was used at doses of 2 and 20mg/kg. In case of a 2mg/kg dose very weak phosphorescence signal was obtained, which is not sufficient for further data calculation using the SPCImage software, therefore, were used TBP dose of 20mg/kg.
The obtained data on TBP phosphorescence intensity and lifetime in the tumor in vivo after intravenous injection of TBP are shown in Figs. 5 and 6.
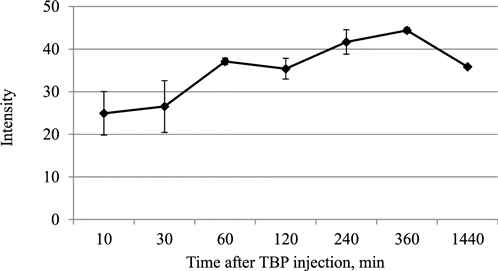
Fig. 5. Intensity of TBP phosphorescence in the S37 tumor of mice after intravenous injection of TBP at a dose of 20mg/kg.
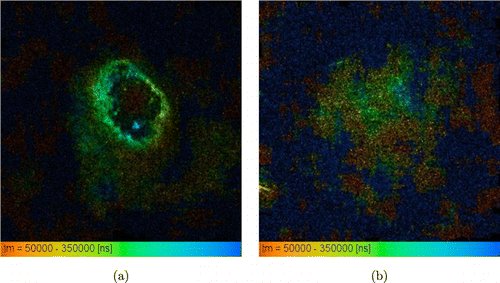
Fig. 6. Time-resolved phosphorescence images of S37 tumors in mice 1 h after intravenous administration of TBP at a dose of 20mg/kg: (a) nodular tumor in which the staining is concentrated along the edge of the node, where vessels are located. In the center, usually, necrosis develops with subsequent formation of a necrotic scab; (b) the diffuse formation is shown.
The obtained data demonstrate that in vivo after intravenous injection of TBP phosphorescence with mean lifetime of 129–287 μs was detected in the S37 tumor within 24h. Over time the phosphorescence intensity of the sensor increased, which is apparently due to the accumulation of the dye in the tumor (Fig. 5).
The distribution of exogenous phosphorescence in the tumor was heterogeneous, which may be a consequence of variability of the oxygen distribution in the tumor, pH (since it is known that the TBP phosphorescence depends on pH),19 and the heterogeneity of the distribution of the dye in the tumor. It also can be noted that there is no phosphorescence in the center of the nodal formation of S37 (Fig. 6(a)), probably due to a necrotic scab formation that quench phosphorescence.
The increase in the TBP phosphorescence lifetime value in tumors (Fig. 7) from 280 to 296μs and from 196± 25 to 282± 14 μs was observed postmortem in case of intratumoral and systemic use, respectively (Figs. 7(a) and 7(b)). In case of the intratumoral application, in muscle postmortem the phosphorescence appeared with the lifetime of 300μs, which was not observed in vivo.
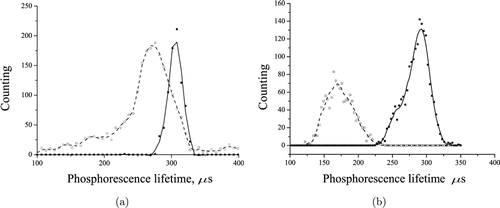
Fig. 7. Phosphorescence lifetime of S37 tumor in mice (a) 1h after intratumoral application of TBP in the concentration of 2mg/mL; (b) 4h after intravenous administration of TBP at the dose of 20mg/kg. The dashed line represents in vivo data, the solid line shows postmortem data.
Thus, postmortem supply of oxygen to tissues is stopped and the phosphorescence of TBP injected into a muscle increase. However, according to our data, the TBP phosphorescence lifetime also increases with decreasing temperature (data not shown or shown elsewhere). After the death of the animals, their body temperature decreased, that could affect the phosphorescence lifetime of the oxygen sensor.
The PDT experiments were carried out. The main mechanism of antitumor effect of which is ischemia of the tumor. The phosphorescence lifetime of “Photosens” sensitized tumors of mice not treated with TBP was 30μs (an order of magnitude lower than τph of TBP), therefore, “Photosens” should not affect the measurement results of TBP τph(Table 3). In all experimental tumors, the TBP phosphorescence lifetime was increased after PDT (Table 3, Fig. 8).
| Phosphorescence lifetime (μs) | ||||
|---|---|---|---|---|
| PDT treatment (left legs) | ||||
| Parameter | Before the PDT | After the PDT | Control, no light illumination, the same mouses (right legs) | |
| Average value | 144 ± 5 | 204 ± 39 | 200 ± 53 | 153 ± 31 |
| % from initial | 100 | 143 ± 29 | 100 | 78 ± 10 |
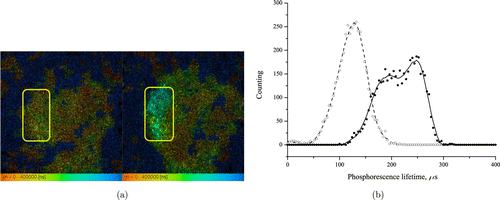
Fig. 8. (a) Time-resolved phosphorescence images of the S37 tumor of the same mouse before (left image) and after (right image) the PDT. (b) Phosphorescence lifetime distribution of the S37 tumor of the same mouse before (dashed) and after (solid) the PDT.
PDT in the used regime caused an antitumor effect: T/C (see 2.4, Eq. (1)) value was 0.22 ± 0.07 and 0.27 ± 0.09 at 1 and 2 weeks after PDT, respectively, i.e., the phosphorescence lifetime increase was associated with the therapeutic effect of PDT. The obtained data indicate that the TBP phosphorescence lifetime increase in the experimental tumor occurs as a result of tumor ischemia under the influence of PDT with the Photosens drug.
4. Conclusion
This work represents a pilot study, which is aimed to determine the conditions for further work on the dynamics of tumor oxygenation determination using phosphorescence lifetime imaging method.
As a result of in vivo studies, it has been shown that after intratumoral or systemic administration of Pd (II) TBP without additional effects to mice, the phosphorescence lifetime of S37 sarcoma varies in the range from 129 to 290μs, which indicates a low oxygen content in the tumor and correlates with the published data on the hypoxia of solid tumors.2
We have observed a heterogeneous distribution of the tumor phosphorescence lifetime in mice treated with TBP that may be due to heterogeneity of the oxygen distribution. Under the influence of the actions that stop the blood supply of the tissues (the sacrifice or PDT), and therefore the supply of oxygen in them, the phosphorescence lifetime of TBP in the tissue of S37 sarcoma increases.
Thus, the results obtained in this study correlate well with data obtained by other researchers using different methods.1,2,3,18,23,24,25,26,27,28,29,30
One of the drawbacks of the TCSPC method used in this study is the relatively long signal acquisition time. In our case, it was 10min for 2 full scans. The rate of measurement of the oxygen consumption by the tumor is important during PDT. It would also be interesting to detect photoinduced hypoxia not only of the tumor, but also in the stroma, or in the surrounding tissue (muscle) in our case, since it is known that damage of the stroma is of primary importance for the realization of the antitumor effect. In addition, there are approaches to PDT dosimetry, in which oxygen content in the tumor is measured.31 An interesting point is the study of oxygen consumption in tumor tissue during PDT and the correlation of the antitumor effect with the consumption of molecular oxygen. It would be also interesting to measure the light dose during the process of PDT in order to determine whether irradiation can be stopped. However, to do this it is necessary to carry out phosphorescence measurements quick enough.
The acquisition time can be reduced by minimization of the scanned area. In addition, the use of sensors with a higher phosphorescence quantum yield can also significantly reduce the measurement time.
Conflict of Interest
Author Vladislav Shcheslavskiy was employed by the company Becker&Hickl GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.


