Optical angiography for diabetes-induced pathological changes in microvascular structure and function: An overview
Abstract
Diabetes mellitus (DM) is a kind of metabolic disorder characterized by chronic hyperglycemia and glucose intolerance due to absolute or relative lack of insulin, leading to chronic damage of vasculature within various organ systems. These detrimental effects on the vascular networks will result in the development of various diseases associated with microvascular injury. Modern optical imaging techniques provide essential tools for accurate evaluation of the structural and functional changes of blood vessels down to capillaries level, which can offer valuable insight on understanding the development of DM-associated complications and design of targeted therapy. This review will briefly introduce the DM-induced structural and functional alterations of vasculature within different organs such as skin, cerebrum and kidneys, as well as how novel optical imaging techniques facilitate the studies focusing on exploration of these pathological changes of vasculature caused by DM both in-vivo and ex-vivo.
1. Introduction
Diabetes mellitus (DM) has become one of the most serious metabolic/endocrinological diseases worldwide.1 With the continuously rising number of DM patients, it has become the third biggest leading cause of mortality around the world in the 21th century.2 The number of DM patients have significantly increased from 108 million to 451 million in the past several decades.3 DM is of two types, namely type-1 diabetes (T1D) and type-2 diabetes (T2D). Though T2D patients occupy the majority of diabetic population, T1D more common in younger people also showed an extremely amazing increase in recent years.4 DM is a kind of metabolic disorder generally characterized by chronic hyperglycemia caused by abnormal insulin resistance or insufficient insulin secretion led by the loss of islets.5 Long-term hyperglycemia will cause chronic injury of vasculatures within different kinds of organs and tissues, leading to the occurrence and progress of various complications associated with abnormal vasculature, such as retinopathy, cardiovascular disease, peripheral vascular disease, cerebrovascular disease and microvascular lesion of skin. Additionally, DM-induced vascular lesion can also significantly increase the onset probability of many neurovascular diseases, such as stroke, vascular dementia or Alzheimer’s disease.6,7,8,9
Among all the complications induced by DM, microvascular lesions within skin, eyes, brain and kidney are the typical complications leading to severe damage to human health.10 For example, microvascular change within the skin is generally considered to be the most common clinical manifestation of DM and may be associated with complications in other internal organs.11 Microvascular change within the eyes is closely related to diabetic retinopathy, which is the leading cause of vision loss for DM patients.12 Additionally, vascular lesions in brain and kidney are the key factors for the development of brain disease and diabetic nephropathy (DN).13,14 Therefore, precise characterization of the DM-derived pathological changes of vasculatures within such organs/tissue can offer valuable insights for clinical studies, thus benefiting in the understanding of the underlying mechanisms of DM-induced vascular complication and design of targeted therapy.
With the rapid development of modern optical imaging techniques, the designed optical angiography not only allows researchers to dynamically observe and measure the blood flow, blood oxygen and vascular permeability of blood vessels within the skin and cerebrum in-vivo,15,16,17,18 but also is capable of acquiring 3D structural information of vascular networks within various tissues with high resolution ex-vivo.19,20,21,22 Up until now, various kinds of advanced optical imaging techniques have been employed for investigating DM-induced pathological changes of vasculatures within different organs/tissues, which significantly facilitate the understanding of the mechanisms of DM-induced vascular complications.23,24
In this review, we briefly introduced the currently available optical imaging techniques for vascular imaging, the changes of vascular structure and function during the progress of several vascular complications led by DM using advanced optical angiography, including microvascular lesion within the cerebrum, skin, as well as the structural changes of glomeruli in diabetic kidneys. In addition, advanced optical imaging techniques used for studying these changes induced by DM were also discussed.
2. Advanced Optical Imaging Techniques for Visualizing Vasculature
With the rapid development of optical imaging technology, a lot of optical tomography techniques have been developed and employed for imaging vasculature within biological specimens at high resolution both ex-vivo and in-vivo, such as confocal and multi-photon microscopies,25,26 optical coherence tomography (OCT)27,28 and optical coherence tomography angiography (OCTA),29,30,31 laser Doppler flowmetry (LDF), laser speckle contrast imaging (LSCI),32 hyperspectral imaging33 and light-sheet fluorescence microscopy (LSFM).34 However, the imaging depth of optical tomography is often limited due to the high scattering characteristic of tissue. In this case, modern optical clearing techniques were proposed and developed to effectively reduce light scattering within the tissue. Nowadays, many optical imaging methods mentioned above are used in combination with optical clearing for enhanced imaging depth and contrast.
Confocal and multi-photon (e.g., two-photon and three-photon) microscopies are powerful optical imaging techniques widely used for the visualization of internal structures within various biological specimens at subcellular level, such as neurons, blood vessels and microglia.35 Notably, they play rather important roles in intravital vascular imaging, for they offer not only high-resolution imaging of blood vessels down to capillaries level ex-vivo, but also dynamic evaluation of vascular structure and function in-vivo. OCT is a noninvasive imaging method that permits investigation of the internal structures of optically inhomogeneous tissues of different types at high resolution.27 OCTA was developed as an extension of OCT imaging technique. OCTA technology relies on detecting the differences in amplitude, intensity or phase variance between sequential B-scans taken at the same location.30 Due to its noninvasive imaging mode, high resolution with a depth-resolved fashion as well as dye-free injection, OCTA has been widely used for in-vivo visualization of the retinal microvasculature under normal and pathological conditions, including age-related macular degeneration (AMD), diabetic retinopathy (DR) and uveitis.20,36,37 LDF is based on measuring the Doppler shift to provide real-time, continuous flow assessment for advanced microvascular imaging.38 LDF is widely used for monitoring the reactive flow of cutaneous microvasculature under various conditions or stimuli.39 LSCI is a powerful method allowing the noninvasive and fast mapping of blood flow with a large field of view. LSCI technique possesses abilities for imaging of the blood flow distribution at high spatial and temporal resolutions, and is widely employed in studies for functional monitoring of blood flows within cutaneous microvasculature.40,41 LSFM was designed to overcome the time-consuming nature of point-scanning methods such as confocal, aiming at imaging tissues with large volume. LSFM generates thin light sheet from the side to uniformly illuminate the specimen and collects the fluorescence from the illuminated plane, enabling volumetric imaging with faster speed, higher signal-to-noise ratio and lower photobleaching compared to point-scanning methods.42 In recent years, LSFM gradually became a key approach for imaging optically-cleared specimens with large volume.43
Though various kinds of advanced optical imaging techniques have been proposed, the imaging quality is reduced due to the high tissue scattering and absorption.44 In the past several years, tissue clearing techniques have emerged as a powerful tool to reduce light scattering and absorption by using various physical and chemical means, making the tissue transparent and thus improving the imaging depth and quality of optical tomography.45 Tissue clearing techniques have two directions, including in-vivo tissue optical clearing methods and ex-vivo tissue optical clearing methods.46 Both clearing methods have been successfully employed for vascular-associated imaging according to different experimental needs. For example, in-vivo optical clearing is mainly used for dynamic monitoring of the blood vessels within the skin or cortex.40,47,48Ex-vivo optical clearing mainly focuses on 3D visualization of intact vasculature in fixed tissues/organs.49,50
The combination of tissue clearing techniques and optical imaging tomography provides an effective method for structural and functional imaging of vasculature. Meanwhile, an image processing pipeline is also required for visualizing and analyzing data acquired by the optical microscope. Generally, it is necessary to analyze the microscopic image on the computer with excellent configuration to carry out procedures such as image compression, segmentation and reconstruction. It is convenient for researchers to use the commercially available software to analyze their data for specific needs, such as Amira, Imaris, Vision4D, etc., but they need to pay an expensive license fee for using them. Additionally, there are also many open-source software available for image analysis, such as BigDataViewer,51 Vaa3D,52 Fiji,53 etc. Among them, Fiji is probably the most widely used platform that can fulfill most demands for processing the image in two dimensions, but is less powerful for 3D reconstruction and visualization of large image datasets. Recently, machine learning has shown great potential in image analysis, permitting robust and efficient information extraction and complex analysis in a wide range of experimental settings automatically.54
3. DM-induced Pathological Changes in Skin Vasculature
3.1. DM-induced changes in skin microvascular structure
Skin is the largest and most accessible organ of the human body, which often served as a model for the study of microcirculation under normal and pathological states.39 Traditional studies have revealed that DM will lead to certain structural changes of skin vasculature. For example, studies have found that the capillaries beneath the nail folds of T1D patients would suffer morphological changes with the course of DM.15 In addition, DM can cause the abnormal structural change of perithelial cells and the apoptosis of endothelial cells within skin vessels.55,56 With the recent development of advanced optical imaging techniques, DM-induced changes in skin microvascular structure are comprehensively studied both in DM patients and animal models.15,16,57
For noninvasive studies on DM patients, Sorelli et al. monitored the perfusion of blood in T1D patients with LDF.58 They found that the skin microvessels of T1D patients had lower perfusion baseline and revealed stronger response to local thermal stimuli than the control group. They also found that arteriosclerosis in T1D patients is highly relevant with uncontrolled blood glucose within one year through pulse decomposition analysis of the LDF data. A similar study was also conducted by Lal and Unni that employed LDF to analyze blood perfusion in the foot in T1D patients.59 They analyzed the changes of microcirculation in diabetic patients by time fractal analysis and found that the Hurst coefficient of diabetic patients was significantly reduced compared with the normal group. A study conducted by Adamska et al. demonstrated that in DM patients with diabetic kidney complication, it is usually accompanied with angiogenesis and vascular maturation.60 The density and maturity of skin microvessels for these patients are significantly reduced compared with patients without diabetic kidney disease.
Many researchers also conducted well studies on DM models. Krumholz et al. utilized photoacoustic imaging techniques to measure the changes of vessel diameter, blood flow velocity as well as oxygen metabolism for both arteries and veins within ear skin in T1D mouse model.16 They found different pathological changes induced by DM between arteries and veins, i.e., for arterial vessels, both diameter and blood flow velocity decreased significantly, however, the diameter of venous vessels decreased and the velocity of blood flow increased obviously (Fig. 1(a)). Additionally, the total hemoglobin concentration and oxygen saturation for both vessel types did not show evident change. Schaefer et al. used intravital fluorescence microscopy to study the changes of morphological and functional parameters of microvessels in the uncoupling promotor-driven diphtheria toxin A chain (UCP1/DTA) mice, aiming at obtaining valuable information for early microvascular deterioration in the early stage of DM.57 UCP1/DTA mice possessed larger diameter of blood vessels but obviously reduced density of functional vessels compared to normal mice, which is largely due to the loss of small vessels with a diameter from 3mm to 12mm. They found obvious tissue hypoperfusion although no significant change was found for blood flow rate of a single vessel, probably due to the decreased vessel density. Moreover, the permeability of vessels within the skins of UCP1/DTA mice was doubled compared with the normal group.
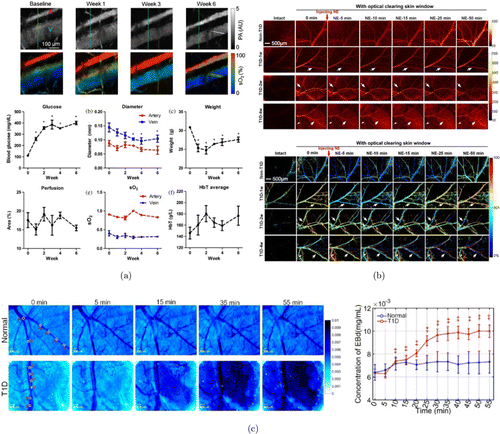
Fig. 1. Examples of DM-induced skin vascular lesions. (a) Structural and functional changes from streptozotocin-induced DM mice for six weeks. Images adapted from Ref. 16. (b) Dynamic monitoring of NE-induced skin vascular blood flow and blood oxygen response at different stages of T1D through optical clearing skin window. Images adapted from Ref. 63. (c) Change of permeability of cutaneous microvessels caused by T1D. Images adapted from Ref. 67.
3.2. DM-induced changes in skin microvascular function
DM not only affects the normal vascular structures with skin, but also leads to obvious dysfunction of skin microvascular environments, causing serious complications that affect the normal life of DM patients.
Among the various complications that happen in the skin of DM patients, diabetic foot ulcer seems to be a kind of pervasive complication caused by vascular dysfunction induced by hyperglycemia. Jan et al. measured the skin blood perfusion by LDF and quantitatively evaluated the metabolic, neurogenic and myogenic responses.61 They found a significant reduction of metabolic, neurogenic and myogenic responses to thermal stimulation, which demonstrated that DM would cause microvascular dysfunction by introducing metabolic, neurogenic and myogenic control disorders. Another study executed by Tehrani et al. comprehensively investigated the relationship between cutaneous microvascular response and microvascular damage in T1D patients by LDF.11 The results showed an obvious reduction in DM patients of the response peak of skin blood flow when Ach and SNP were introduced. Additionally, patients with microvascular disease induced by DM showed a further lower response than patients without microvascular complications, which revealed that the vascular response in DM patients is closely related to the degree of microangiopathy. Argarini et al. visualized the skin microvessels within the instep of DM patients by OCT.62 They measured the structural changes in vessel diameter and density as well as functional changes in blood flow velocity after thermal stimulation of the foot skin. They found that DM patients with foot ulcer showed less changes in vessel diameter and density than patients without foot ulcer under heating, the blood flow velocity was less changed as well.
Several research groups also conducted studies on DM mouse model to investigate the skin microvascular dysfunction. Feng et al. investigated the response of blood flow and blood oxygen to noradrenaline (NE) within healthy and T1D mice at different durations by LSCI and hyperspectral imaging.63 They overcame light scattering by in-vivo skin optical clearing techniques, which can significantly increase the imaging quality by matching the refractive index (RI) within the skin tissue.64,65,41 The distribution of blood vessels under the skin can be clearly visualized, the blood flow and blood oxygen of observed vessels can also be finely detected and calculated (Fig. 1(b)). They found that the blood flow of venous and arterial vessels decreased significantly in T1D mice and did not recover after injection of NE. Additionally, the degree of arterious blood oxygen reduction had weakened with the development of T1D, probably due to the change of α1-adrenergic receptor expression. To further investigate the dysfunction of skin vessels led by T1D, Feng et al. injected SNP and Ach to normal and T1D mice for quantitative analysis of the response of skin blood flow and blood oxygen.66 The results revealed that short-term (1–2 weeks) T1D showed no obvious influence on cutaneous arteriovenous blood flow in response to injection, however, the recovery of blood flow after injection with SNP was ahead of schedule in three- and four-week T1D mice. Additionally, nearly all T1D mice showed an obvious decrease of the SNP-induced response of blood oxygen compared with normal controls. These studies demonstrated that T1D would lead to the disturbance of both blood flow and blood oxygen response.
Besides the abnormal response of blood flow and blood oxygen, DM can also lead to the increased permeability of skin vessels. Schaefer et al. observed fluorescent dye leakage due to increased vascular permeability induced by DM in skin-fold chamber.57 Feng et al. quantitatively evaluated the Evans Blue (EB) dye leakage of blood vessels within T1D mice skins through optical clearing window and reflective microscopy imaging.67 They found that no significant change occurred in the concentration of EB dye inside and outside the blood vessels at different time points for healthy mice, however, the concentration of EB dye outside the blood vessels was obviously increased for T1D mice 10 min after injection, indicating that T1D will alter the vessel permeability in skin tissue (Fig. 1(c)).
4. DM-induced Pathological Changes in Retinal Vasculature
Diabetic retinopathy is a typical microvascular complication induced by DM.68 Statistics have showed that DR has become the leading cause of vision loss among the working age group worldwide, representing a rather heavy burden on global healthcare.12 In recent years, optical imaging techniques have become popular tools for studying vascular changes of retina led by DM.36
The pathological changes of foveal avascular zone (FAZ) are considered to be a reliable metric in both normal eyes and eyes with DR.69 Early studies have investigated the vascular changes of FAZ by fluorescein angiography (FA) and found that the area of FAZ obviously increases with the progress of DR.70 However, methods like FA or indocyanine green angiography (ICGA) only allow for 2D visualization of retinal vasculature with limited resolution. Additionally, typical studies have revealed that FA can only detect a fraction of vasculature within retina when comparing FA to histology examinations.71 OCTA has been successfully proved to replicate findings achieved by FA in the entire retinal vascular structures.72 Several studies have done comparison of FA and OCTA; the results have shown that OCTA allows better discrimination of FAZ and microvasculature than FA.72,73 Choi et al. employed OCTA to study diabetic eyes from no retinopathy to proliferative diabetic retinopathy (PDR); they successfully found retinal microvascular abnormalities including vascular remodeling adjacent to the FAZ and enlarged FAZ.74
Besides pathological changes in FAZ, microaneurysms (MA) are another landmark finding in DR and are proved to be closely associated with progression of advanced disease stages until vision loss.75 Studies showed that OCTA can detect approximately 60% of MA identified on FA due to the decreased blood blow within the focal dilations of retinal capillaries.72 However, OCTA was still able to detect some microaneurysms that were not observed by FA. Other abnormalities of retinal vasculature led by DM were visualized by OCTA such as areas of retinal nonperfusion, reduced capillary density and increased vessel tortuosity.76 The vascular changes discovered on OCTA are closely related to the increasing DR severity.77 Many studies have repeatedly identified that there existed a negative association between vessel density and increasing DR severity at both the superficial and deep capillaries.78,79
In other words, the DR induced by DM includes several findings of particular relevance to clinical practice, namely vascular remodeling of FAZ, enlarged FAZ and increase of capillary tortuosity and dilation, which can be effectively detected by OCTA during the progression of DM.
5. DM-induced Pathological Changes in Cerebral Microvasculature
5.1. DM-induced alteration of cerebrovascular structure and brain blood barrier permeability
DM-induced long-term hyperglycemia is tightly associated with kinds of neurovascular diseases that seriously affect the function of cerebral microvessels.80 Many studies have revealed that microvascular lesion is closely related to cerebral complications induced by DM.81,82 Therefore, it is essential to study the DM-derived abnormality of cerebrovascular structure and function to better understand the pathogenesis of various complications.
Presently, changes of microvascular structure and function within the brain induced by DM are mainly investigated via animal models. Traditional studies have revealed that the ultrastructure of vascular endothelial cells changed obviously in cerebral vessels with the onset of DM.9 In the acute phase of hyperglycemia, cerebral microvascular endothelial cells appeared with swelling with apparent cracks within the endothelial junctions.83 The progress of DM will result in morphological changes of brain–blood barrier (BBB), which manifested as basement membrane thickening, calcium deposition in microvascular wall, capillary deformation and microthrombus.80 The resolution of traditional histopathology is enough to distinguish changes of microvascular structure, however, it is difficult to observe the blood vessels in a large field of view and obtain functional information. Modern optical techniques can help visualize the structural and functional changes of cerebral vessels down to capillaries level, which provide essential tools for studying DM-related cerebral complications.6,7,14,84,85,86
To explore the potential mechanism of ischemic stroke caused by DM, Kelly-Cobbs et al. employed optical microscopy to study the changes of middle cerebral arteries in diabetic rats.87 They found that the thickness and wall/lumen ratio of cerebral vessels were significantly increased compared to healthy rats, which had been further proved to be related to increased MMP-2 and reduced MMP-13 activities. Prakash et al. studied the remodeling patterns of cerebral neovascularization in two different T2D models by confocal imaging.88 They labeled the brain vasculature by perfusion of FITC-conjugated dextran, and the sectioned brain slices were counterstained with isolectin B4 to distinguish vessels not labeled by FITC. They quantitatively measured the structural parameters of vessels including vascular volume, microvessel/macrovessel ratio, vessel tortuosity and branch density by reconstruction of fluorescent-labeled vasculature acquired by confocal microscope. They found a disproportional increase of total vascular density, volume and surface area in cortex and striatum in the GK model, which was rather different from the control group. Additionally, the introduction of metformin treatment to control glucose right after the onset of DM could prevent the increase in vascular density (Fig. 2). As for Leprdb/db mouse model, the results showed no difference in vascular density and nonperfused/perfused ratio between diabetic model and normal mice, but an increase in microvascular volume and area.
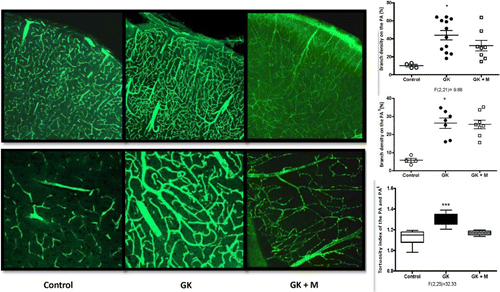
Fig. 2. Pathological changes of cerebrovascular architecture induced by DM. Structural changes of cerebral vessels in normal mice, DM mice and DM mice with treatment, including increased branch density, lumen diameter and tortuosity. Images adapted from Ref. 88.
BBB is a special anatomical structure with selective permeability between brain microvessels and brain tissue, which can allow nutrient transportation while isolating harmful substances into the central nervous system.89,90 BBB is the key structure to maintain the homeostasis of the brain environment. A lot of studies have revealed that hyperglycemia caused by DM could lead to dysfunction of BBB in different DM animal models.80,82,91 In recent years, modern optical tomography provides a powerful tool to measure the changes of BBB permeability for cerebral vasculature and abnormity of BBB-related proteins caused by DM.92 Stranahan et al. examined the effects of protein kinase Cβ (PKCβ)-mediated cerebrovascular breakdown on BBB integrity in different types of DM mice.93 The leakage of injected fluorescein into the neuropil was obvious in the hippocampal regions of brain sections from db/Veh mice and db/Enz mice. They also used Enzastaurin — a PKCβ inhibitor — to successfully survive the BBB disruption. These results revealed that the up-regulation of PKCβ induced by DM played an important role in BBB breakdown. Yu et al. investigated various kinds of tight junction proteins that are closely related to the structure and function of BBB by immunolabeling and immunohistochemistry in DM mice, including ZO1, VE-cadherin and Occludin.94 They found that the labeling for several molecules was fragmented and discontinuous in DM mice, revealing that DM would cause the down-regulation of BBB-associated structural proteins. Additionally, the results showed that the staining became more intact and continuous after recombinant FGF21 (rFGF21) administration compared with the untreated group, which demonstrated that rFGF21 treatment could reverse the DM-induced BBB permeability change. Rom et al. observed increased BBB permeability and memory loss related to hyperglycemia in both T1D and T2D mice models; they found memory deficits, endothelial injury and neuroinflammation that happened in DM models were closely associated with BBB disruption.95
5.2. DM-induced changes in cortical microvascular blood flow/oxygen response to drugs
Given that DM is a chronic metabolic disease with different developmental stages,96 the damage to microvascular function led by DM is also different in the DM course. Therefore, it is important to monitor the dynamic changes in the cerebrovascular dysfunction of patients or animal models in different durations of DM.
Recently, several studies have dynamically monitored the cerebral microvascular responses by optical imaging and skull optical clearing techniques using DM mouse models.46 Different from open-skull window or thinned-skull window, in-vivo skull optical clearing technique could overcome light scattering of the intact skull, providing a convenient and surgery-free skull window for fine visualization of cortical neurovascular structure and function through the transparent skull.97,98 With the assistance of this advanced technique, Feng et al. visualized vascular response to the drugs by a home-built laser speckle/hyperspectral two-mode imaging system. They recorded and analyzed the relative changes of blood flow and blood oxygen before and after the injection of SNP and Ach (Fig. 3(a)).66 They found that the initial decrease almost disappeared after the injection of SNP in T1D mice at different stages, which was rather different from non-T1D mice. In particular, the degree of blood flow change induced by SNP became unobvious for the three- and four-week T1D mice (Fig. 3(b)). Similarly, the diminished blood flow response after injection of ACh was also observed in T1D mice. The results also showed that the relative change of blood oxygen during the development of T1D increased directly. Furthermore, the SNP-induced change of blood oxygen in cerebral vessels in one–four-week T1D mice was larger than that observed in normal mice. In addition, the ACh-induced change of blood oxygen only occurred in veins for the changes in artery almost disappeared in one–four-week T1D mice. These results revealed that T1D could lead to abnormal changes in cerebrovascular blood flow and blood oxygen responses at its early stage and these changes would become more and more serious as the DM progressed.
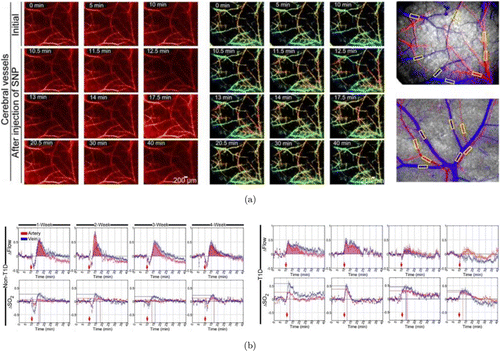
Fig. 3. Measurements of dynamic responses of blood flow and blood oxygen saturation in non-T1D and T1D mice. (a) Typical maps of the blood flow and blood oxygen saturation for cerebral vessels imaged through an optical clearing skull window before and after the injection of SNP. (b) Statistical analysis of time-lapse data showing the relative changes in cerebral vascular blood flow and the corresponding blood oxygen saturation that occurred in arteries (red) and veins (blue) after the injection of SNP in different stages of T1D. Images adapted from Ref. 66.
6. Visualization of DM-Induced Structure Changes of Kidney Microvasculature
6.1. Traditional histopathology of abnormal kidney microvasculature induced by DM
Diabetic nephropathy is one of the most serious chronic microvascular complications for diabetic patients.99,100 In the past several decades, with the rapidly increasing number of DM patients, the incidence of DN is also gradually increasing and has become one of the leading causes of end-stage renal disease (ESRD).1,101 Statistics showed that nearly one third of T1D patients and half of T2D patients are suffering from this microvascular complication.13,102,103 Although most DM patients with ESRD are with T2D, T1D still takes up a large number of new ESRD cases and shows a growing increment in recent years. Therefore, comprehensive investigation of pathogenesis and therapies of DN is rather important.
Persistent hyperglycemia led by DM will lead to serious damage and disruption of the renal cellular architecture and microvasculature at single glomerulus level.104 Researchers have made great efforts in understanding the pathophysiology of DN to date.105,106,107,108 Presently, microvascular structure change within diabetic kidneys has been investigated via traditional clinical observations from DM patients or diabetic animal models with the assistance of optical or electron microscope (Fig. 4). Generally, the major microvascular pathology of DN is characterized by the glomerular lesion induced by hyperglycemia.109 In both T1D and T2D, microstructural injury is noted in many structures including glomeruli, arterioles, tubules and the interstitium within diabetic kidneys, which is closely related to several typical clinical manifestations such as progressive albuminuria and increased blood pressure.110,111 The glomerular lesions induced by T1D mainly affect the structures of the renal tubules, podocytes and arterioles, especially after several years of onset of DM.112,113 Current studies have revealed that glomerular lesion led by DM is mainly characterized by glomerular basement membrane (GBM) thickening, mesangial widening and nodular lesions.105 Clinical studies have showed that the first detectable change of glomerular lesion after the occurrence of T1D might be the GBM thickening, which is often recognized as capillary and tubular basement membrane thickening by microscopic imaging.107,114 Transmission electron microscopy (TEM) is often used to detect these morphological changes of DN at this stage. Optical tomography can observe obvious glomerular hypertrophy due to the early structure changes induced by DM.105 The width of GBM tends to increase linearly during the progress of T1D. Mesangial expansion is another typical lesion of DN, which is primarily caused by an incrassation in mesangial matrix during DN development. Nodular lesions occur at the periphery of the glomeruli, which can be well exhibited by periodic acid–Schiff-positive staining that defines globular structure while exaggerating diffuse lesions. It is considered to be associated with development of Kimmelstiel–Wilson nodules which could be seen at the later stage of DN by optical microscopy.115 Additionally, there is also an increasing recognition of lesions like glomerular endothelial injury, podocyte impairment and glomerulotubular junction abnormalities during the progress of DN.116,117,118,119
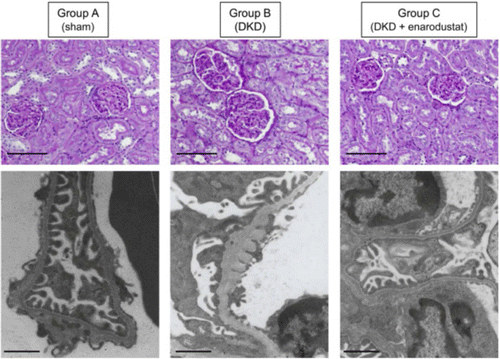
Fig. 4. Traditional renal pathologies in streptozotocin-induced DM rats. Representative pathological images including periodic acid–Schiff staining images and electron microscopy images are shown. Images adapted from Ref. 146.
Accurate characterization of glomerular structural damage during the progress of DN must rely on clinical visualization and analysis of the renal biopsy, which is the gold standard for diagnosis and target therapy for DN.100 However, it may be difficult to have access to perform large-scale clinical trials using the patient’s renal tissues. Recently, with the development in microengineering technology, it is possible to create organ-on-chip microdevices lined with glomerular microtissues to mimic the microarchitectures of kidneys, which provide new tools for researchers to study DN with 2D cell culture in dish or glomerulus-on-chip microdevice.120 Up to now, this model is successfully used to study the changes of glomerular barrier in phenotype expression, barrier integrity and permeability in the presence of high-glucose medium.
6.2. Tissue optical clearing for 3D imaging of the overall vascular structures of normal and DM kidneys
Though histopathological examination acts as a gold standard for characterizing glomerular lesion induced by DN, the above studies are all based on 2D images that lack spatial structure information, thus they are not able to investigate the overall changes of the intact kidney vasculature as well. The rapid development of advanced optical imaging and fluorescent labeling techniques makes it possible to acquire 3D tissue structures with high resolution, however, the imaging depth is limited by the high light scattering within the tissue.44,47 Tissue optical clearing techniques are developed to solve this problem by overcoming the RI mismatch within the tissue via different physical and chemical means, which can facilitate the understanding of the complex biological architectures of whole organs and organisms.121,122 In recent years, various tissue clearing techniques have been proposed for studying the various biomedical events.123 They can be principally divided into three categories: solvent-based clearing methods, which are represented by DISCO-series124,125,126; aqueous-based clearing methods, which are represented by Scale series and clear, unobstructed brain imaging cocktails and computational analysis (CUBIC) series127,128; and hydrogel-based clearing methods, which are represented by CLARITY series.129,130 The mechanisms and related applications of advanced tissue clearing techniques have been reviewed extensively in recent years.45,131,132,133
Among the numerous tissue clearing methods, many of them have already been proved to be capable for 3D imaging of vascular structures within mouse kidneys. For example, 3D imaging of solvent-cleared organs (3DISCO) developed by Ertürk et al. is a typical solvent-based clearing method which has been confirmed to be useful for imaging the vasculature of mouse kidneys labeled by fluorescent-conjugated tomato lectin.124 DISCO with superior fluorescence-preserving capability (FDISCO) developed by Qi et al. can be used for 3D imaging and reconstruction of kidney vasculature labeled by CD31 antibodies, quantification of glomeruli was also performed.125 Immunolabeling-enabled DISCO (iDISCO) method proposed by Renier et al. was recognized as a powerful whole-mount immunolabeling protocol, which can be applied to image the entire kidneys labeled by anti-Aquaporin2 for collecting ducts and anti-Nephrin for glomeruli, respectively.134 Ethanol-ECi was used for 3D imaging of the CD31-labeled glomeruli within intact mouse kidneys with nephrotoxic nephritis, quantification of total glomerular number and size was performed based on a customized image processing pipeline.135 Polyethylene glycol (PEG)-associated solvent system (PEGASOS) method was employed for imaging the transgenic-labeled kidney vasculature (Fig. 5(a)).136 Recently, Zhao et al. rendered the adult human kidney transparent and performed 3D histology with fluorescent dyes for centimeters depth by their developed SHANEL protocol.137 CUBIC clearing protocol imaged and reconstructed the whole mouse kidneys labeled by nucleic acid dyes (Fig. 5(b)).138,139 Matryba et al. proposed an optimized perfusion-based CUBIC protocol to image the intact rat kidneys stained by propidium iodide (PI).140 Recently, the CUBIC protocol was also upgraded for volumetric imaging of human renal samples.139 Additionally, CUBIC method was also especially optimized for kidney samples, named as CUBIC-kidney, realizing the whole-mount immunolabeling and dual-color imaging of sympathetic nerves and vessels in the entire mouse kidneys for healthy and injured.141 Recently, Zhu et al. developed the MXDA-based aqueous clearing system (MACS) clearing protocol and reconstructed the glomerular trees within mouse kidneys labeled by lipophilic dye DiI.142 Additionally, after the acquisition of 3D image stacks of vasculature by confocal or light-sheet microscope, an image analysis pipeline should be employed using commercial or open-access software143,144,145 or custom-made codes for segmentation and quantification of glomeruli.135
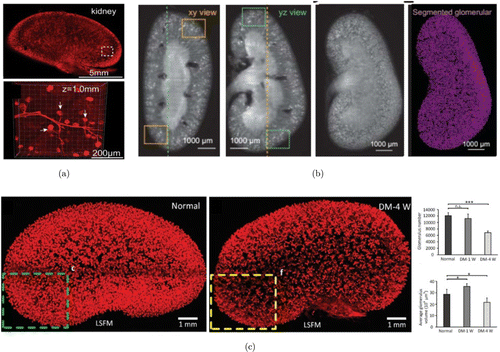
Fig. 5. The 3D pathology of glomeruli in normal and DM kidneys by tissue clearing pipeline. (a) The 3D imaging of transgenic-labeled glomeruli and blood vessels in normal kidneys cleared by PEGASOS. Images adapted from Ref. 136. (b) Segmentation of glomeruli within intact normal kidneys cleared by CUBIC. Images adapted from Ref. 139. (c) Reconstruction and quantitative analysis of DiI-labeled glomerular tufts and vessels in normal and diabetic kidneys by MACS. Images adapted from Ref. 142.
Although a growing number of tissue clearing techniques have been developed and employed for 3D imaging and analysis of kidneys vasculature, however, most of them mainly focused on morphological structures of normal kidneys. Up to now, there are only limited studies involving characterization of glomerulus lesion of T1D-induced DN using tissue optical clearing techniques. Zhu et al. constructed the T1D model with continuous administration of alloxan, they then labeled the vascular structures in normal and diabetic mouse kidneys by perfusion of DiI and performed 3D pathology of glomeruli with MACS clearing and light-sheet imaging.142 The overall glomerulus trees within the entire normal and diabetic kidneys were observed as well as the fine structures of the capillary tufts of individual glomerulus. Quantification of the glomerulus number and volume of entire kidneys was also performed (Fig. 5(c)). They found that the glomerulus number did not change in the short term of DM, but the average glomerulus volume increased. As for long-term DM, both the glomerulus number and volume were obviously reduced, which revealed that long-term T1D would lead to both individual tuft defects and glomerular loss. Hasegawa et al. utilized T1D rodent models induced by streptozocin (STZ) or alloxan and demonstrated the net effects of HIF stabilization on energy metabolism in diabetic kidney with the assistance of CUBIC-kidney clearing protocol.146 They also performed 3D quantifications of glomeruli in the kidney labeled by anti-podocin antibodies for normal, diabetic and treatment groups. They found that T1D-induced glomerular loss and renal pathological abnormalities (e.g., GBM thickening) in the early stages of DN could be partially reversed by treatment with enarodustat.
The above-mentioned tissue clearing studies paved the way for facilitating the pathological investigation of microvascular structure changes in T1D-induced DN. However, application of tissue clearing in DN-related studies is still primitive and has limitations. For example, these studies utilized T1D animal models induced by STZ or alloxan, where there still existed many pathological differences compared with clinical patients. Thus, there is still a long way to go for tissue clearing to facilitate the histopathological understanding of DN. It is expected that the advanced optical imaging techniques are expected to be coupled with the histopathology techniques, which will significantly promote the comprehensive investigation of nephrology studies.
7. Conclusions
As a kind of microvascular-associated disease induced by hyperglycemia, diabetes mellitus has become one of the most serious metabolic disorders with an increasing global challenge for worldwide healthcare. Modern optical imaging techniques provide indispensable tools for investigation of the pathological changes of vascular networks down to capillaries level, and they have been widely used in studying DM-derived microvascular complications. In this paper, we have shortly reviewed the typical chronic vasculature complications of typical organs induced by DM including skin, cerebrum and kidneys. Additionally, the pathological changes including structure and function related to these complications are able to be comprehensively investigated by advanced optical imaging techniques.
Many studies have revealed that DM will lead to structural and functional lesions of skin and cerebrum vasculature. Modern optical imaging techniques can realize the dynamic monitoring of blood flow, blood oxygen and vascular permeability within the skin and cerebrum in-vivo. Concerning skin vascular lesions, DM can lead to various skin microvascular dysfunctions, including reduced vascular maturation, lower blood perfusion, changed vascular diameter and density, abnormal response to drugs which constrict or dilate blood vessels and enhanced vascular permeability. All of these may be potential diagnostic targets of DM. As for DM-induced cerebrovascular lesions, DM can influence brain function by causing cerebral vascular dysfunction from the aspects of structural changes, BBB breakdown and abnormal response. It is associated with increase of vessel wall, vessel tortuosity, branch density and decrease of the BBB-related proteins, leading to enhanced BBB permeability. In addition, DN is one of the most serious chronic microvascular complications of DM. The morphological changes of glomerulus caused by DN can be clinically investigated by traditional histopathology via light microscopy or TEM. Recently, the optical clearing techniques have been used for the 3D reconstruction of neural and vascular structures within the entire kidneys combined with modern optical imaging techniques. Several optical clearing methods have already been developed and used for studying the glomerular structure changes induced by T1D in three dimensions.
Optical imaging techniques can provide rather valuable structural and functional information on DM-induced vascular lesions at high spatial and temporal resolutions, facilitating the understanding of various complications. However, these techniques also have certain limitations. For example, the photobleaching and phototoxicity caused by confocal and multi-photon microscopies will lead to potential injury of biological samples. Potential disadvantages of OCTA seem to be its limited field of view, inability to view dye leakage to assess vessel function and increased risks of artifacts. As for the tissue optical clearing techniques, ex-vivo tissue clearing agents are usually aggressive and toxic, which may cause damage to both samples and researchers. The clearing agents for in-vivo studies are nontoxic, however, many negative effects still exist with additional treatment time or high concentration, such as irritation, edema, alteration of vessel morphology and even vessel blocking. These drawbacks are expected to be resolved in the future studies.
Additionally, some DM patients will suffer from the hyperosmolar hyperglycemic state which is characterized by acute decompensation of DM with pronounced hyperglycemia (usually plasma glucose >35mmol/L), high plasma osmolarity and severe dehydration but without ketosis and acidosis. Some patients even go into shock in a very short time which can be life-threatening. The systemic dehydration could cause abnormal changes of vascular structure and function within a wide range of organs, which can serve as a potential target for early diagnosis. For example, the dehydration level of the skin may be closely related to the change of blood flow within the skin during the progress of hyperosmolar hyperglycemic state, which can be detected by various optical angiography techniques. Additionally, tissue optical clearing techniques are expected to be used to overcome the high scattering character of human skin for high imaging contrast. Therefore, the combination of such techniques holds great potential for the early diagnosis and assessment of dehydration of DM patients suffering from the hyperosmolar hyperglycemic state.
In summary, the recently developed optical imaging techniques have been proved to offer the essential tools for the comprehensive exploration of pathological changes of many kinds of DM-induced vascular lesions within different tissues/organs, which will greatly facilitate the understanding of the pathogenesis underlying various DM-induced vascular complications.
Conflicts of Interest
The authors declare that there are no conflicts of interest relevant to this paper.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFA0700501), the National Natural Science Foundation of China (Grant Nos. 61860206009, 81870934, 62105113 and 81961138015), China Postdoctoral Science Foundation Funded Project (Nos. BX20200138, BX20190131, 2021M691145 and 2019M662633) and the Innovation Fund of WNLO.


