Research on the relationship between reduced scattering coefficient and intracranial pressure in brain edema model
Abstract
Intracranial hypertension is a serious threat to the health of neurosurgical patients. At present, there is a lack of a safe and effective technology to monitor intracranial pressure (ICP) accurately and nondestructively. In this paper, based on near infrared technology, the continuous nondestructive monitoring of ICP change caused by brain edema was studied. The rat brain edema models were constructed by lipopolysaccharide. The ICP monitor and the self-made near infrared tissue parameter measuring instrument were used to monitor the invasive intracranial pressure and the reduced scattering coefficient of brain tissue during the brain edema development. The results showed that there was a negative correlation between the reduced scattering coefficient (690nm and 834nm) and ICP, and then the mathematical model was established. The experimental results promoted the development of nondestructive ICP monitoring based on near infrared technology.
1. Introduction
Traumatic brain injury (TBI) is a threat to human life because of its high disability rate and mortality rate.1,2 Usually, brain injury will lead to brain edema and then intracranial pressure (ICP) increases.3 It is important to obtain the ICP changes of patients with brain edema quickly and accurately, which is the premise of timely and effective rescue. ICP monitoring has important clinical significance for observing the patient’s condition and making effective treatment plan. The invasive ICP monitoring technology has the characteristics of accurate measurement and strong reliability, considered as the gold standard.4,5 In clinical practice, the pressure sensor is placed under dura mater, ventricle or brain substance, and the doctor can directly achieve the pressure on the cranial cavity exerted by the contents of the cranial cavity.6 However, after the pressure sensor probe is placed into brain tissue, long-term monitoring may cause cerebral hemorrhage or increase brain infection risk. Therefore, it is difficult to monitor neurosurgical patients based on invasive ICP monitoring technology for more than seven days, which hinders clinicians to detect patients’ condition timely and effectively.
In recent years, nondestructive ICP monitoring technology has been developed and researchers have found some new methods.7,8 Studies showed that when intracranial hypertension occurred, the blood flow capacity in the blood vessels decreased and energy metabolism slowed down, leading to the electrical signal conductivity decrease between the brain cells. Based on flash visual evoked potential technology, the conductivity can be detected, shown as the change of signal waveform.9 However, the technology is affected by the blood glucose concentration, age and electrolyte disorder, leading to its small application range and low accuracy. Bioelectrical impedance method can also be used in the nondestructive ICP detection.10 The changes of brain tissue led to the changes of its impedance characteristics, and the physiological and pathological information can be obtained by analyzing the impedance change information. The technology has the characteristics of safety and low cost, but its resistance accuracy is affected by time and pressure and it is not sensitive to small lesions.
Long-term, accurate and safe ICP monitoring technology is still under study. It is found that the near-infrared light (690–900nm) has good penetrability for tissues and body fluids.11,12 The near-infrared light signal emitted from the tissue carries the structure and chemical information of the tissue. Near infrared spectroscopy (NIRS) technology has the characteristics of low analysis cost, nondestructive analysis and real-time in vivo.13 At the same time, patients can contact near-infrared light for a long time without discomfort because of nonionizing radiation. The near-infrared spectroscopy measurement method can be used in the study of breast cancer and brain function (noninvasive), also intraoperative tissue parameters monitoring (minimally invasive).14 Considering the advantages of NIRS technology, the long-term and continuous nondestructive ICP monitoring caused by brain edema could be realized.
Some researchers monitored the cerebral oxygen saturation of neurosurgical patients by near infrared monitoring system.15,16 It was found that there were significant changes in cerebral oxygen saturation before and after oxygen inhalation, and there were significant differences in cerebral oxygen saturation change under different flow oxygen inhalation conditions, which indicated that the NIRS can be used to monitor and evaluate the blood oxygen supply capacity of patients with TBI.17 In the previous research, Liu et al. studied the optical parameters changes of rats’ brain tissue during the development of brain injury based on NIRS.18 TBI causes cerebral blood flow and brain volume, and then affects the optical parameters changes of brain tissue. The optical parameters and spectrum of brain tissue measured at this time had a certain relationship with ICP. Therefore, it is feasible to judge the changes of ICP according to the changes of optical parameters. It is of great significance to study the relationship between the specific near infrared parameters and ICP.
In this paper, nondestructive monitoring of ICP changes caused by brain edema was studied and clinical application were discussed. The animal brain edema model was constructed by lipopolysaccharide. Then, Codman ICP monitor and the near infrared tissue parameter measuring instrument were used to monitor the invasive ICP and reduced scattering coefficient of rats’ brain tissue, respectively. The reduced scattering coefficient and ICP in blank group, experimental group and control group were compared and the relationship model between the reduced scattering coefficient and ICP parameters was established. The negative correlation between the reduced scattering coefficient and ICP in the process of brain edema was proved by animal experiments, which provided experimental basis for clinical nondestructive ICP monitoring.
2. Material and Method
2.1. Experiment equipment
In the experiment, ICP monitor system and near infrared tissue parameter measuring instrument were applied. The Codman ICP monitor (82-6635, Codman & Shurtleff, Inc, USA) was used to detect the ICP parameters of brain tissue in vivo. The pressure monitoring range is from –50 to 250mm Hg and the measurement accuracy of ICP is ±1mm Hg. The near infrared tissue parameter measuring instrument was used to achieve optical parameters of animal brain tissue. It is composed of a computer, a light source, a Y-type fiber probe and a fiber spectrometer, shown in Fig. 1. The computer software has abundant functions, such as optical parameters calculation and data storage in real time. The output power of the light source (HL 2000-hp, Ocean Optics) is 7W and the wavelength range is 360–1700nm. The fiber spectrometer (USB2000, Ocean Optics) and the self-made fiber probe are used for collecting optical signal and the diameter of the probe is 600μm. The Y-type probe had two optic fibers with diameter of 200μm, which were connected to light source and optic spectrometer, respectively.

Fig. 1. The near infrared tissue parameter measuring instrument (1: computer, 2: light source, 3: fiber spectrometer, 4: fiber probe).
Reduced scattering coefficient was selected to study the relationship with ICP parameters. The detail in reduced scattering coefficient calculation was given in the previous research.19 It was found that the slope (700–850) of the spectrum was relevant to the reduced scattering coefficient in some wavelength: Reduced scattering coefficient = A*exp (B*Slope). Slope represents the slope (700–850) of the spectrum. Reduced scattering coefficient in some wavelength (e.g., 690nm and 834nm) can be calculated by different A and B values.
2.2. ICP and reduced scattering coefficient of brain edema model measurement experiment
Lipopolysaccharide (LPS o55: B5, sigma l-2880) was used to build animal brain edema model. The Sprague Dawley rats (SD rat, Rataceae and Rattus, male, 300g, about 10 weeks old) were randomly divided into three groups, including blank group (n = 10), experimental group (n = 10) and control group (n = 10). In blank group, the rats were only separated by the common carotid artery without injecting. In experimental group, the rats were injected LPS (LPS crystal: normal saline = 1mg: 1ml) into the common carotid artery.20 In control group, the rats were injected the same amount of 0.9% normal saline. The rat brain edema model construction is as shown in Fig. 2.
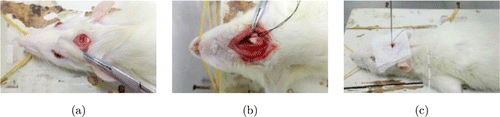
Fig. 2. Brain edema model construction in rats: (a) Skull drilling, (b) left common carotid artery separation and (c) probes insertion.
The rats were intraperitoneally injected with 0.35ml/kg 10% chloral hydrate. After anesthesia, they were fixed on the fixator in prone position. First, drill holes in the skull of rats for ICP probe and fiber probe insertion and monitoring. Specifically, after disinfection, an incision (2cm long) was made at the midline of the skull and the skin was fixed by tissue forceps. Then, we drilled a hole (a diameter of 0.2cm) on the skull and kept the dura mater intact. We covered the wound with a gauze soaked in normal saline preparing for probe insertion.
Second, internal carotid artery separation and endotoxin injection were performed. The rats were fixed on the fixator in supine position. After disinfection, an incision (2.5cm long) was made at the midline of the neck and the cervical skin was fixed by tissue forceps. Then the left common carotid artery was separated, the left internal and external carotid arteries were dissociated. The left external carotid artery was clipped and injected with LPS (0.05ml/100g) or saline in different groups. After pressing for hemostasis, we released the left external carotid artery clamp. We covered the wound with a gauze soaked in normal saline.
Finally, the rats were fixed on the fixator in prone position again. After the dura mater was removed, the ICP probe and fiber probe were inserted into the brain with a depth of 0.5cm. The experimental diagram is shown in Fig. 3. The near infrared optical parameters and invasive ICP signal were measured at the same time and the total monitoring time was 2h.
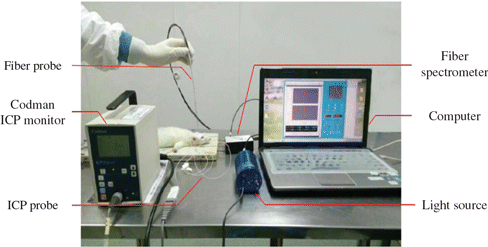
Fig. 3. Brain edema model experiment diagram.
2.3. Experiment data processing
After data collection, statistical analysis was performed by the Analysis of Variance test. If the results showed that there was a significant difference between groups, the data was further processed. Brain injury caused by brain edema is a slowly changing physiological process. In order to observe the tissue parameters during brain edema more effectively, the data collected in the experiment were smoothed and averaged in every minute. Then, we obtained the change curve of ICP and reduced the scattering coefficient in different brain edema states of rats. In order to further analyze the relationship between the reduced scattering coefficient and ICP parameters, the experimental data were normalized and fitted. The data is normalized by the ratio to the initial value. After normalization, the initial value is 1.
3. Results
Analyses of Variance test were done and the results indicated that the experimental data had significant differences within and between different groups (P < 0.05). The change curves of ICP and reduced scattering coefficient in different brain edema states of rats are shown in Fig. 4. In blank group (Figs. 4(a) and 4(b)), the reduced scattering coefficient (690nm and 834nm) curve changed little during 0–75min. In 75–95min, it dropped by around 1cm−1, then became stable. The ICP values changed slightly within 2h, and the range was 0.5mmHg. In experimental group (Figs. 4(c) and 4(d)), the reduced scattering coefficient (690nm and 834nm) curve changed slightly during 0–30min. In 30–80min, it had a significant downward trend and then became stable. The ICP values had an obvious upward trend during 30–80min. In 0–30min and 80–120min, they changed with a smaller range. The asterisks meant that the data showed significance (P < 0.01) from baseline. In control group (Figs. 4(e) and 4(f)), the reduced scattering coefficient (690nm and 834nm) values began to decrease from 15min and maintained for 15min, and then slowly increased until 120min.
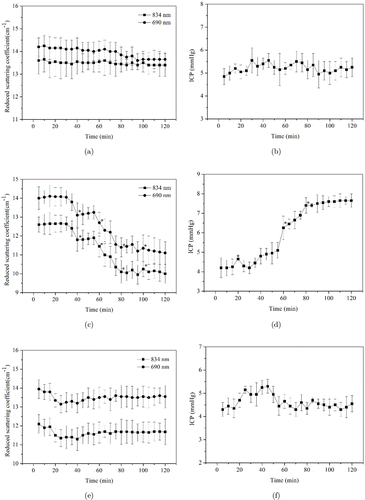
Fig. 4. The reduced scattering coefficient and ICP values of brain tissue from brain edema rats in different groups: (a) Reduced scattering coefficient values in blank group. (b) ICP values in blank group. (c) Reduced scattering coefficient values in experimental group. (d) ICP values in experimental group. (e) Reduced scattering coefficient values in control group. (f) ICP values in control group: ∗means that the data shows significance (P < 0.01) from the baseline.
Comparing the changes of ICP and reduced scattering coefficient in blank group, experimental group and control group, it could be found that the changes of ICP caused by brain edema were basically consistent with that of the reduced scattering coefficient. In order to further study, the ICP and reduced scattering coefficient in different groups were normalized and analyzed.
Figures 5(a)–5(e) are the normalization curves of reduced scattering coefficient (834nm in Figs. 5(a) and 5(b) and 690nm in Figs. 5(c) and 5(d)) among different groups and the two curves have the similar law of change. The reduced scattering coefficient curve in the blank group showed a slow downward trend. In control group, it decreased at 15min and increased gradually during 15min, then kept a very small change. However, in the experimental group, it decreased rapidly after 35min, and the decrease range was much larger than that in the blank group and the control group. In each figure, the asterisks meant that the data showed significance (P < 0.01) between the two groups.
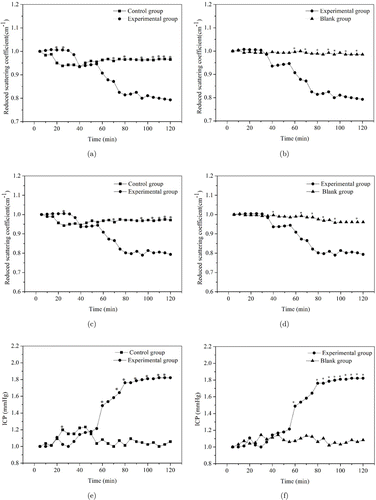
Fig. 5. The normalized reduced scattering coefficient and ICP values of brain tissue from brain edema rats: (a), (b) Reduced scattering coefficient (834nm) values. (c), (d) Reduced scattering coefficient (690nm) values. (e), (f) ICP values: ∗means that the data shows significance (P < 0.01) between the two groups.
The normalization curve of ICP among different groups is shown in Figs. 5(e) and 5(f). In blank group, the ICP curve had no obvious upward or downward trend. In the control group, ICP curve first increased, then remained unchanged for 15min, and then decreased gradually. In experimental group, the ICP values increased rapidly after 35min, and the increase range was much larger. The results show that the changes of reduced scattering coefficient caused by brain edema were basically consistent with that of ICP. Therefore, the ICP in brain edema model could be evaluated by observing the reduced scattering coefficient change.
In order to further analyze the relationship between the reduced scattering coefficient and ICP parameters, we fitted the normalized data. The normalized reduced scattering coefficient (834nm in Fig. 6(a) and 690nm in Fig. 6(b)) and ICP data were selected for fitting. The experimental results show that the fitting degree of the curve is very high, which can be used to build the mathematical model of the reduced scattering coefficient and ICP parameters in brain edema model.
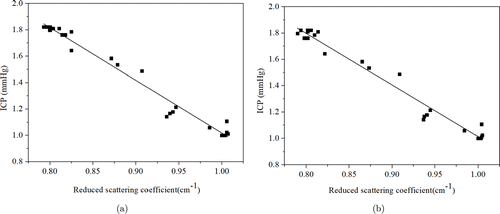
Fig. 6. The fitting curve of normalized ICP values versus reduced scattering coefficient values from brain edema rats: (a) Reduced scattering coefficient (834nm) values, (b) Reduced scattering coefficient (690nm) values.
The fitting formula of data in Fig. 6(a) is as follows :
4. Discussion
Intracranial hypertension caused by brain edema is a common pathological syndrome in neurosurgery, which poses a great threat to the safety of patients.21 At present, there are few safe and effective ICP monitoring devices which can be used for long-term bedside monitoring. NIRS technology has strong penetrability in biological tissues and strong sensitivity for biological tissue differences.22 It is harmless to human body and easy to operate, which can be used for long-term bedside monitoring. In this paper, the ICP and near infrared optical parameters of brain edema model were monitored in real time and analyzed. Specifically, the TBI model caused by brain edema was established by endotoxin reagent. By monitoring the minimally invasive ICP and reduced scattering coefficient of brain tissue, it was found that there was a negative correlation between the reduced scattering coefficient (690nm and 834nm) and ICP, then the mathematical model of ICP and reduced scattering coefficient was established. By observing the reduced scattering coefficient change of brain tissue, we could indirectly obtain the ICP change, and then evaluate the development of brain edema.
In this paper, the animal model of brain edema was made by injecting the endotoxin reagent. Different from the commonly used brain edema model made by external force impact, the brain edema model made by endotoxin injection had less complications, mainly angiogenic brain edema. The toxicity of endotoxin is weak.23 When endotoxin enters the host, it can cause fever, microcirculation disturbance, endotoxic shock and disseminated intravascular coagulation. A large amount of endotoxin enters the host blood, the body produces immune response and releases interleukin and tumor necrosis factor TNF-α, Serotonin, prostaglandins and other bioactive substances, which directly or indirectly damage capillary endothelial cells, increase the permeability of blood–brain barrier, accumulate a large amount of liquid in cell space, cause extracellular edema and the decrease of reduced scattering coefficient of brain tissue. The construction of other brain edema models in animals should be discussed in the next step.
NIRS technology was applied in our experiments with low near-infrared radiation. However, some research indicated that infrared radiation could not be considered as totally innocuous to human skin.24 It was considered that more than a certain infrared irradiation dose would increase the collagen degrading enzyme and matrix metalloproteinase, resulting in the imbalance of dermal extracellular matrix. Schroeder found that the human skin fibroblasts could withstand irradiation doses up to at least 1200 J/cm2.25 How to choose an appropriate near infrared radiation dose in the experiment is still a difficult problem. In the future study, we will analyze the effects of different irradiation doses on the experimental results.
The relationship of ICP and reduced scattering coefficient in a specific brain edema model were studied in this paper. The brain edema induced by lipopolysaccharide is mainly extracellular edema.26 However, acute hypoxia also causes brain edema, which belongs to intracellular edema. There is a big difference in the size of brain tissue cells between the two types of brain edema.19 The change of reduced scattering coefficient caused by intracellular edema is not clear, which can be explored in the next study. The study will provide reference for clinical diagnosis and judgment of brain edema type. Due to the lack of enough clinical data in this paper, the mathematical model of ICP and reduced scattering coefficient under different wavelengths will be built in the future, which will help to promote the application of NIRS technology in clinical brain injury monitoring.
5. Conclusion
This study found that the NIRS technology had the advantages of large tissue penetration depth and long-term monitoring. In this paper, the relationship between the reduced scattering coefficient and ICP in brain edema model based on NIRS were studied. The rat brain edema model was established by lipopolysaccharide. The ICP monitor and the self-made near infrared tissue parameter measuring instrument were used to measure the ICP and the reduced scattering coefficient of brain tissue, respectively. We obtained the change rule of ICP and reduced scattering coefficient in the development of brain edema and built the corresponding mathematical model. Moreover, the change rule of reduced scattering coefficient and ICP in different states of clinical patients were discussed, which verified the experimental results. The experimental results promoted the development of nondestructive ICP detection technology.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by National Major Scientific Instruments and Equipment Development Project Funded by National Natural Science Foundation of China (81827803 and 81727804) and the National Natural Science Foundation of China (61875085).


