Overview of novel nanobiosensors for electrochemical and optical diagnosis of leukemia: Challenge and opportunity
Abstract
Leukemia is one of the ten types of cancer that causes the biggest death in the world. Compared to other types of cancer, leukemia has a low life expectancy, so an early diagnosis of the cancer is necessary. A new strategy has been developed to identify various leukemia biomarkers by making blood cancer biosensors, especially by developing nanomaterial applications so that they can improve the performance of the biosensor. Although many biosensors have been developed, the detection of leukemia by using nanomaterials with electrochemical and optical methods is still less carried out compare to other types of cancer biosensors. Even the acoustic and calorimetric testing methods for the detection of leukemia by utilizing nanomaterials have not yet been carried out. Most of the reviewed works reported the use of gold nanoparticles and electrochemical characterization methods for leukemia detection with the object of study being conventional cancer cells. In order to be used clinically by the community, future research must be carried out with a lot of patient blood objects, develop non-invasive leukemia detection, and be able to detect all types of blood cancer specifically with one biosensor. This can lead to a fast and accurate diagnosis thus allowing for early treatment and easy periodic condition monitoring for various types of leukemia based on its biomarker and future design controlable via internet of things (IoT) so that why would be monitoring real times.
1. Introduction
Blood cancer is a type of cancer caused by abnormal growth of white blood cells, causing the body’s weakness to fight infection.1 In the United States, there are 1,629,474 blood cancer patients and the number of cancer sufferers will increase by 9.4% in 2023.2 There are several types of blood cancer that have different characteristics, effects on other organs, and different treatments. Blood cancer is a liquid cancer so that it quickly spreads to other organs of the body.3,4
Based on the previous case, leukemia is generally divided into 3, namely acute leukemia caused by proliferation of immature white blood cells and is devided into acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML), chronic leukemia which is caused by clonal proliferation of early progenitor hematopoietic stem cells that can be caused by bone marrow abnormalities and devided into chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML), and hairy cell leukemia (HCL) caused by the presence of lymphocytes B which has projections that resemble small hairs, but this case is very rare.5,6,7 Blood cancer has a low 5-year life expectancy, which is 31.8%.8 Thus, early detection of specific types of blood cancer is important to do. Complete Blood Count (CBC) is the most commonly used conventional method for the detection of blood cancer, but it is necessary to re-test peripheral blood (PB) and bone marrow (BM) to detect the specific type of blood cancer.3
The development of biosensors for various specific types of leukemia has been carried out using acoustic, electrochemical and optical detection methods with surface modification of the biosensor.9,10 The development of biosensors for early detection of cancer with nanostructures has been widely developed with various apatamers to obtain sensitive and selective results for certain types of blood cancer.10,11 The biosensor consists of a sample, a transducer, and an electronic system. In a blood cancer detection system, the sample may consist of DNA, RNA, blood from leukemia patients, and leukemic cells. Meanwhile, transducers are divided into bioreceptors and electrical interfaces. Bioreceptors function as identifiers and bind samples with an electrical interface so that they can detect specific biomolecules. Bioreceptors for blood cancer can be DNA and RNA or commonly called aptamers. Systems on transducers for detecting biomaterials are divided into electrochemical, acoustic, calorimetric, and optical. Meanwhile, the electronic system in the biosensor consists of a signal amplifier, signal processor, and signal displayer.
Nanotechnology can be utilized as a way to obtain a very sensitive blood cancer detection system. By using nanomaterials as transducers or amplifiers in biosensor systems, highly sensitive biosensors can be built.9,12 With the increasing demand of innovations in nanomaterials for blood cancer biosensors, it is important to compare the method or type of characterization of various nano modes to obtain optimum sensitivity and selectivity for detecting blood cancer. The type of nano mode with various types of nanomaterial that has been developed for various types of diseases is nanoparticles, nonetheless, various modes of nanostructures have also been developed, such as nanorods, nanotubes, nanoflakes, etc. with various types of characterization methods such as electrochemistry and optics. On the other hand, to the best of our knowledge, there are no recent reviews that summarize and compare the simultaneous use of multiple nanomodes in blood cancer detection applications. In this present article review, we overview the latest innovation of nanotechnology in biosensors application, particularly, electrochemical- and optical-based biosensors. It started from the discussion of basic understanding of nanomaterials for biosensors, especially for blood cancer detection. The prospect and challenges also explained together with plausible strategy on how to increase the feasibility of nanomaterials for biosensors. We believe that the present artcile review will pave the way the gap of real implementation of nanomaterial for biosensors, and open the opportunity in development of new nanomaterials for blood cancer detection purposes.
2. Recent Nanomaterials for Leukemia Detection
The life expectancy of leukemia patients is low compared to other types, so early diagnosis is necessary.13 Clinical diagnosis is currently carried out with blood testing and bone marrow testing is added to determine the specific type of leukemia.3 Detection of leukemia with various specific biomakers can be a key solution for the early detection of specific leukemia.10 Nanomaterials can function as bioreceptors, transducers, and amplifiers so as to increase the sensitivity of biosensors because nanomaterials have attractive properties to biological molecules, have a large surface area-to-volume ratio for binding samples, and have good electrical and mechanical properties.9,14,15,16 Although many biosensors have been developed, the detection of leukemia by using nanomaterials with electrochemical and optical methods is still little done compared to other types of cancer biosensors. Even the calorimetric testing methods for the detection of leukemia by utilizing nanomaterials have not yet been carried out.10 To enhancing of the biosensor, surface modification is fulfilled with nanomaterials. This nanomaterial can function as a biomolecule binder to increase the sensitivity of the biosensor. Figure 1 shows a biosensor design scheme for directional cancer detection by utilizing nanomaterials.
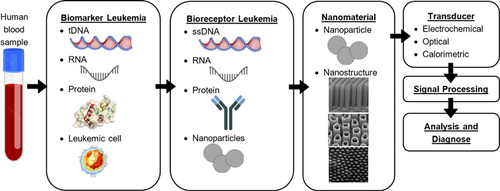
Fig. 1. Biosensor scheme using nanomaterials to detect leukemia, figure adopted with permission from Ref. 17; copyright @ 2021 Molecules, Ref. 18; copyright @ 2019 Frontiers in Materials, and Ref. 19; copyright @ 2012 J. Mater. Chem.
Many researches with nanomaterials are more developed for electrochemical biosensors when compared to optics. Although currently other types of biosensors have been developed, such as acoustics and calorimetry, the use of nanomaterials for the fabrication of these biosensors for the detection of leukemia has never been carried out. Research conducted by Hao et al. in 2013 has developed an acoustic method by testing the Shear Horizontal Surface-Acoustic-Wave (SH-SAW) for the detection of Jurkat and K562 type leukemia cells with a sensitivity of 1000cells/mL by utilizing acoustic wave propagation which causes changes in the frequency and phase of the waves due to the presence of acoustic waves and mass change.20 In addition to the SH-SAW method, there is also a Mass-sensitive quartz crystal microbalance (QCM) method and a thickness shear mode method (TSM) for biosensor characterization that utilizes the viscosity and affinity of the sample and electrode interactions.10,21 However, this type of sensor has not been developed for the detection of leukemia by utilizing nanomaterials as its constituents.
Compared with acoustics and calorimetry biosensors, optical and electrochemical biosensors for leukemia have been more widely developed. This is due to its higher sensitivity and specificity. The optical and electrochemical biosensor can detect low levels of biomarkers associated with leukemia, enabling early diagnosis and monitoring of the disease. This biosensor can also provide faster results than traditional diagnostic methods.22,23 This rapid detection with a minimum sample volume and enabling non-invasive and portable development is essential for initiating early treatment, which can significantly improve patient outcomes.24,25
Electrochemistry is a branch of chemistry that studies the relationship between electrical and chemical changes caused by the flow of electric current.26 Electrochemical methods are often used for various applications, one of which is as a method of testing biosensors. Several types of electrochemical methods for testing leukemia biosensors using nanomaterials are cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), Differential pulse voltammetry (DPV), Anodic stripping voltammetry (ASV), square-wave voltammetry (SWV), Photoelectrochemistry (PEC), and chronoamperometry.14,27,28,29,30,31 Like biosensors in general, electrochemical biosensors also require surface preparation that allows interaction between the sample, bioreceptor, and transducer. In general, sample preparation that can be carried out for electrochemical leukemia detection using nanomaterials is shown in Fig. 2.
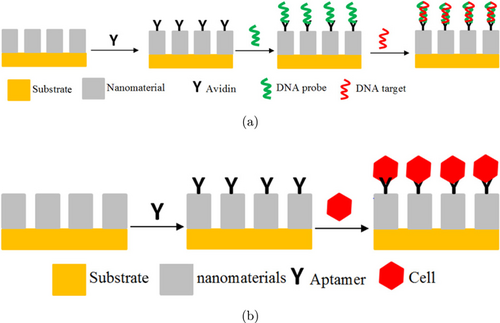
Fig. 2. Schematic illustration of electrochemical leukemia detection using nanomaterials with (a) DNA target and (b) leukemic cells target.32,33
Electrochemical testing for the detection of leukemia is more common. By utilizing samples in the form of nucleic acid, cells, and enzymes, it can be seen that some studies show a decrease in reduction and oxidation peaks, and an increase in impedance equivalent to an increase in sample concentration.27,34 However, a study conducted by Zhang et al. in 2018 showed the opposite result due to the addition of more AuNPs above the sample and showed high sensitivity.35 When compared to the sample preparation method, application of nanobiosensors with clinical testing, target detection using blood is simpler without requiring DNA extraction than nucleic acid. However, the sensitivity value is higher using nucleic acid because of the specific bonds between the chains. Figure 3(a) shows the working scheme of the biosensor for electrochemical detection.
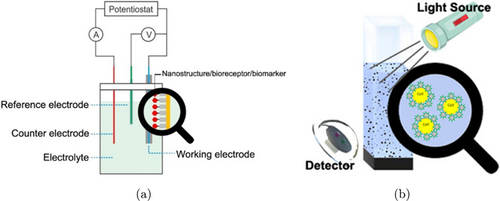
Fig. 3. Illustration of working biosensors for (a) electrochemical and (b) optical detection.
Apart from nanomaterials as biosensors detected electrochemically, nanomaterials can also be used for optical leukemia biosensors. Spectroscopy is a branch of science that deals with measuring the spectrum produced when materials interact with electromagnetic radiation.36 Optical characterized leukemia biosensor can work specifically on a type of leukemia due to the presence of several components. Samples that can be used for optical detection of leukemia are leukemic cells, DNA, RNA, and enzymes. Bioreceptors that can identify specific samples that can be used for optical leukemia detection are DNA probes, nanoparticles, and antibodies. Optical characterization methods that can be used for the detection of leukemia based on research that have been carried out are fluorescence resonance energy transfer (FRET), surface-enhanced Raman scattering (SERS), Surface plasmon resonance (SPR), fluorescence, flow cytometry, colorimetry, Fourier-transform infrared spectroscopy (FTIR), and chemiluminescence.37,38,39,40 In general, sample preparation that can be carried out for optical leukemia detection using nanomaterials is shown in Fig. 4.
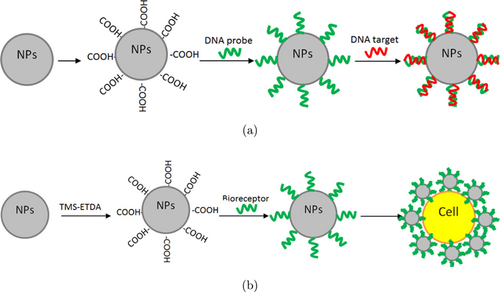
Fig. 4. Schematic illustration of optical leukemia detection using nanomaterials with (a) DNA target and (b) leukemic cells target.41,42
In optical testing, spectroscopic methods can identify the bonds that occur between biosensors and samples by utilizing bioreceptors.43,44,45,46 Figure 3(b) shows the schematic reflection of working truth of biosensors for the detection by optical approach. The presence of bonds between nanomaterials, bioreceptors, and also samples or detection targets can cause the formation of intensity peaks at certain wavelengths in the FTIR, SERS, and SPR tests. In addition, an increase in the concentration of the detection sample bound to the nanomaterial can increase the fluorescence intensity and decrease the intensity of the FTIR, SERS, and SPR test results.37,38,39,40 Banerjee et al. 2021 shows that the sensitivity obtained with bulk materials is lower compared to nanostructures because the surface to volume ratio of the nanostructures used to bind biomolecules is greater. In addition, there is no decrease in biocompatibility and energy on the surface of the nanomaterial.16 The role of nanomaterials as transducers for biosensors with optical detection methods needs to be taken into account for many further developments. Studies on optical biosensors using nanomaterials, especially with 1-dimensional (1D) mode or quantum dot materials, can have high characteristic fluorescence properties. 1D nanomaterials also have a high level of selectivity, capable of forming hydrogen bonds and electrostatic interactions with detection targets which can be confirmed through TEM, FT-IR and zeta potential test data.47,48,49
From the literature study used, most of the types of nanomodes used are nanoparticles. This is because the preparation and synthesis of nanoparticles is easier when compared to other types of nanomaterials, such as nanorods and naotubes.40,50,51 The different types of nanomaterials can be shown in Fig. 5. Then, the results of studies on leukemia biosensors still mostly use DNA and cell cultures as detection target or biomarker, but several studies have used patient blood samples to identify the performance of the biosensor.52,53,54 In addition, non-invasive nanobiosensors with optical methods are also being developed, such as research conducted by Bordbar et al. in 2021.15
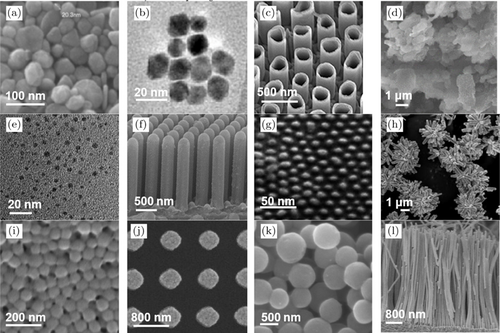
Fig. 5. Microstructure from SEM test results for each type of nanomaterial, including (a) Nanoparticle (NPs); figure adopted from Ref. 55 with permission; copyright @ 2008 Korean Journal of Chemical Engineering, (b) Quantum dots (QDs); figure adopted from Ref. 56 with permission; copyright @ 2014 Physics, Simulation, and Photonic Engineering of Photovoltaic Devices III, (c) Nanotube (NTs); figure adopted from Ref. 18 with permission; copyright @ 2019 Frontiers in Materials, (d) Nanocomposite; figure adopted from Ref. 57 with permission; copyright @ 2019 Materials, (e) Nanocluster; figure adopted from Ref. 58 with permission; copyright @ 2017 Microchimica Acta, (f) Nanorods; figure adopted from Ref. 17 with permission; copyright @ 2021 Molecules, (g) Nanodots; figure adopted from Ref. 19 with permission; copyright @ 2012 J. Mater. Chem., (h) Nanoflower; figure adopted from Ref. 59 with permission; copyright @ 2013J. Mater. Chem. A, (i) Nanobeads figure; adopted from Ref. 60 with permission; copyright @ 2021 Nanomaterials, (j) Nanodisk; figure adopted from Ref. 61 with permission; copyright @ 2019 Nanoscale, (k) Nanosphere; figure adopted from Ref. 62 with permission; copyright @ 2015 Nanomaterials, (l) Nanowire; figure adopted from Ref. 63 with permission; copyright @ 2019 Journal of Semiconductors.
2.1. Biosensor based on nanoparticle (NPs)
Nanoparticles are materials that have a size between 1 to 100nm. Nanomaterials have good chemical and physical properties and are attractive to biomolecules but their optical properties depend on their size.64 Thus, nanoparticles are good candidates as biosensors both electrochemically and optically. There are several types of nanoparticle used for optical and electrochemical biosensors for leukemia detection, including AgNP, AuNP, PtNP, magnetic nanoparticles, ZnNP, ZnONP, SiNP, rGONP, MgFe.37,51,52,65,66,67,68,69 Among all types of nanoparticles, AuNP is the most common type of nanoparticle used as a leukemia biosensor. This is due to the ease with which it binds to biomolecules to form Au-S bonds.70
2.2. Biosensor based on quantum dots (QDs)
Quantum dots (QDs) are semiconductor nanoparticles that have a size between 1.5 and 10.0nm. QDs have high size-dependent optical properties, photostability, extinction coefficient, and brightness, and large Stokes shifts.71 Thus, QDs can be utilized for both optical and electrochemical biosensors. The types of QD materials that have been developed as leukemia biosensors are Ag2S, CdTe, graphene (GR), and C3N4.27,33,41,72 CdTe is most widely used to detect leukemia because in optical testing, CdTe QDs can reduce background emission from acceptors. There is also a combination using AuNPs to obtain a high sensitivity, which is 1cell/mL.73 QDs have better advantages compared to other types of materials when used as sensors. Although metal QDs are less biocompatible than bio-based QDs, metal-based quantum dots have special advantages, both as optical and electrochemical sensors. Research conducted by Goswami et al. in 2022 showed a high activation of fluorescence due to the structure of graphene QDs caused by an emission mechanism caused by aggregation and also an increase in current conduction, sensitivity and stability of the biosensor.49,74
2.3. Biosensor based on nanotube (NTs)
Nanotubes are cylinders made of nanostructures and have photo-sensitive properties. Nanotubes have advantages, including having a much higher conductivity than copper, not reacting with other types of atoms, and changing electrical properties depending on their bond with other molecules.75 One of the nanotubes can be fabricated using the electrodeposition method by adjusting the voltage and time of deposition.18 Nanotubes have been widely used in biosensor applications, one of which is as a leukemia biosensor.34,41,54,76,77,78,79 In addition to carbon nanotubes, there are also polyaniline nanotubes that are used as leukemia biosensors. Carbon nanotubes are effectively used for leukemia biosensors because carbon nanotubes having good electrical conductivity and electrocatalytic properties so as to improve electron transfer reactions and biosensor sensitivity.77 In addition, the combination of carbon nanotubes with AuNPs and ZnONPs can also improve the performance of the electrode for detecting leukemia because it can expand the bonding surface with the sample and increase cell affinity transduction.34,54,79 From the literature study that has been carried out, these nanotubes are still used for the detection of leukemia by electrochemical methods, but can also be used for optical testing because of their photosensitive properties.75
2.4. Biosensor based on nanocomposite
Nanocomposite is a combination of two or more phases of material of the order of nanometers.80 There are several studies that have developed nanocomposites as a constituent of blood cancer biosensor.28,29,81,82,83 The performance of nanocomposite as a biosensor depends on the interaction between the nanocomposite and the sample. Nanocomposite-based sensors have better sensitivity, electrical conductivity, selectivity, response, and performance than other nanomaterials84 research conducted by Khoshroo et al. in 2021 showed a high electrocatalytic activity in CuS-graphene nanocomposites when compared to graphene nanoparticles alone. Thus, the nanocomposite can produce high signal amplification. This is due to the synergistic bond between CuS and graphene so that the charge transfer rate between bioconjugates and nanocomposites is also high and can be used as an electrochemical signal amplifier.82
2.5. Biosensor based on nanocluster
Nanoclusters are microscopic particles that have a size smaller than 2nm. These nanoclusters have quasi-continuous energy levels so that they may have photoluminescence properties so that they are good for use as transducers in optical testing.85 This nanocluster is also capable of being a component of leukemia biosensors, both for optical testing methods by fluorescence and electrochemical testing by CV and EIS.15,41,86
2.6. Biosensor based on nanorods
Nanorods (NRs) are nanomaterials that have a maximum diameter of 100nm and a ratio between height and diameter of 1 to 20. Nanorods can be fabricated using a template according to the ratio of height and diameter.87 When compared to bulk thin films, nanorods have the same function in terms of sensing but have a larger surface area.88 A good arrangement of nanorods also has a good capacity in charge transfer. In applications as optical and electrical leukemia nanobiosensors, ZnO and Au type nanorods have been developed. The combination of the Au g-C3N4 composite nanorod was proven to be able to increase the sensitivity of the electrode.28,50,89
2.7. Biosensor based on nanodots
Nanodots are a type of nanoparticles classified specifically with a particle size between 2 to 20nm.90 The development of nanodots has been carried out for the detection of electrochemically detected leukemia DNA. Research by Ardakani et al. in 2019 also combined carbon nanodots with AuNP and obtained a fairly good sensitivity, which was 0.26pgmL−1.83,91
2.8. Biosensor based on nanoflower
Nanoflower is one type of nanomaterial that has the most complex morphology but has a very high surface energy compared to other types of nanomaterial modes.92 Nanoflower is also capable of being a constituent of leukemia biosensors, especially in electrochemical biosensors.78,93 Although the fabrication process is quite complex, the sensitivity of this nanoflower is quite promising. Au nanoflower has a sensitivity of 10cells/ml, while AuNP has a sensitivity of 79cells/ml to detect K562 cells.93,94
2.9. Biosensor based on nanobeads
Nanobeads are nanoparticles that have a diameter of 80 and 200nm.69 Currently, the utilization of nanobeads is used for optical detection of leukemia by binding to cell surface antigens and blocking the binding of some fluorescent labels. The performance of nanobeads in detecting B-lymphoblast cells using the flow cytometry method is quite good, which has a sensitivity of up to 95%.95
2.10. Biosensor based on nanodisk
Nanodisk is a type of nano material that is able to bind integral membrane proteins and membrane proteins so as to increase stability and even distribution of proteins.96 Research conducted by Pang et al. utilizes nanodisks to bind quantum dots to detect CCRF-CEM cells so that they have a fairly low detection limit, which is 20cells/mL.33
2.11. Biosensor based on nanosphere
A nanosphere shape is a nanomaterial structure that has a size between 10 to 200nm.97 The connection of trimetallic nanospheres consisting of PtAuRh can significantly increase the specific surface area and achieve catalytic activity. The trimetallic nanosphere can be utilized for monitoring metastases and tumor prognosis, especially by electrochemical methods.98
2.12. Biosensor based on nanowire
In general, nanorods and nanowires have similarities in their morphology. However, the ratio between length and diameter has a value of more than 20.87 Ag and carbon nanofibers have been used as a constituent of leukemia electrochemical biosensors because they have good electrochemical properties and three-dimensional networks for electrochemical reactions.99,100,101
The abovementioned nanomaterials are commonly used for biosensing application. The compilation of the previous report of electrochemical and optical-based biosensor using several types of nanomaterials is shown in the Table 1. We also add some important information from those nanomaterials for electrochemical and optical-based biosensor, such as leukemia types, detection methods, detection target, etc.
| Leukemia type | Biosensor type | Detection method | Detection target | Bioreceptor | Type of nanomaterials | Selectivity/linear range | Limit of detection/sensitivity | Ref |
|---|---|---|---|---|---|---|---|---|
| AML | Opt | FRET | HL-60, L292, RAW264.7 | Ald, A1094 | Ag2S quantum dots | N/A | 3Sb/k | 72 |
| AML | Opt | SERS | DNA of THP-1 | colloidal hya-AgNPs | AgNP | 82.35% | 52 | |
| AML | Opt | SPR | HL-60 | VEGF, IL-8 | Gold nanorods | 0.5nm/(ng/ml) | 5.9, 7.2ng/ml for VEGF, IL-8 | 89 |
| AML | Opt | Flrsc | HL-60 | KH1C12 aptamer | Gold nanoclusters | 10–200cells/μL | 86 | |
| AML, CML | Opt | SERS | HL-60, K562 | nanoparticle | AgNP | 98.3% | 102 | |
| CML | Opt | SPR | BCR/ABL fusion genes | DNA probe | Au@PtNP | 1pM to 100nM | 190fM | 103 |
| CML | Opt | Colorimetry | mRNA | ssDNA | AuNP, AgNP | 10ngμL−1 | 66 | |
| CML | Opt | FRET | BCR/ABL fusion gene | DNA probe | CdTe quantum dots | 1.0×10−91.25×10−7M | 1.5×10−10M | 41 |
| CML | Opt | Flrsc | RNA of K562 | DNA probe | Magnetic Nanoparticles | <1fM | 39 | |
| CML | Opt | Colorimetric | miR-21, miR-155 | DNA probe | AuNP | less than one ng/μL | 51 | |
| ALL | Opt | SERS | Jurkat, THP-1, MONO-MAC-6 | Nanoparticle | AgNP | 99% | 40 | |
| ALL | Opt | FC | CCRF-CEM | Sgc8 aptamer | semiconductor quantum dots | 71.6% | 104 | |
| ALL | Opt | FC | B-lymphoblast cell | CD10, CD19 antibody | Nanobeads | 95% | 95 | |
| ALL | Opt | Colorimetry | Blood volatile organic compounds | Nanoparticle | AuNP, AgNP | 93.2% | 15 | |
| AL | Opt | Flrsc | TdTase | HpDNA | Silver nanoclusters | 1–35mUmL−1 | 0.8mUmL−1 | 105 |
| PML | Opt | SPR | PML/RAR α gene | DNA probe | Dendritic nanostructure | 0.72pM, 0.65pM for L, S subtype | 106 | |
| ALL | Opt | FC | MOLT-4 | anti-CD5 antibodies | ZnO nanorod | 3 to 128cells/mm2 | 3cells/mm2 | 50 |
| Not specific | Opt | FTIR | lysozyme | MIP | Carbon dots (CDs) | 0.001–0.01mg/mL | 0.55μg/mL | 73 |
| Not specific | Opt | FTIR | 8-hydroxy-2′-deoxyguanosine from WBC | Py1, Py2 | TiO2Ag, TiO2Au NPs | 98.00% | 10–14molL−1 | 107 |
| Not specific | Opt | Chemiluminescence | Lysozyme | Aptamer | AgNP | 40fM–300fM | 44.6femtomolar | 108 |
| AML | Elc | CV, EIS, SWV | KG1a | CD123 antibody | Graphene QDs, AuNP | 1–25cells/mL for 10–4μA | 1cell/mL | 27 |
| AML | Elc | CV, EIS | FLT3 gene | DNA probe | AMWNTs/AuNP | 0.1–1000pM | 0.1pM | 34 |
| CLL | Elc, Opt | SWV, FTIR | DNA target | capture DNA, T4DNA ligase | Au nanorods/g-C3N4 composite | 0.6nM to 6.4nM | 20pM | 28 |
| CLL | Elc | DPV | ROR1 protein | monoclonal Ab of ROR1 | AgNP, LDH/rGO nanocomposite | 0.01–1pgmL−1 | 10agmL−1 | 29 |
| CLL | Elc | EIS | ZAP70 gene | ZAP70 probe | AuNP | 2.0×10−14−10−9mol/L | 4.0×10−15molL−1 | 109 |
| CML | Elc | EIS | DNA target | DNA probe | PANI-MoS2 nanoflower | 10−6M–10−17M | 3×10−18M | 78 |
| CML | Elc | CV, EIS | cDNA | ssDNA | Gold nanoclusters | 0.1fM–10pM | 0.030fM | 53 |
| CML | Elc | CV, EIS | K562 | Peptide | Nanoparticle | 98% | 8cells/mL | 110 |
| CML | Elc | EIS | DNA target | DNA probe | Carbon nanotube | 1fM–1μM | 1fM | 77 |
| CML | Elc | CV, EIS | BCR/ABL gene | DNA probe | carbon nanotubes, ZnO-NPs | 6.94aM to 694fM | 0.039aM | 54 |
| CML | Elc | CV, EIS | K562 | monoclonal antibodies | Carbon nanotube | 19cells/mL | 76 | |
| CML | Elc | CV, EIS | K562 | T2-KK1B10 capture aptamer | AuNP | 1×102–1×0107cells | 79cells/mL | 94 |
| CML | Elc | CV, EIS | K562, DNA | ssDNA, con A | CdTe Quantum dots | 1.0×102–1.0×107cellsmL−1 | 60cellsmL−1 | 111 |
| CML | Elc | CV | K562 | Aptamer | Au Nanoflower | 0.1–5.0×104cellsmL−1 | 10cells/mL | 93 |
| CML | Elc, Opt | EIS, FTIR | DNA target | DNA probe | ZnONP | 10−16M–10−6M | 10−16M | 69 |
| CML | Elc | ASV | K562 | Antibody | CdS QDs | 1ng/mL to 250ng/mL | 0.5fmol | 31 |
| CML | Elc | PEC | DNA of b3a2 | DNA probe | CdTe QDs | 0.1fM to 5pM | 25.6aM | 30 |
| CML | Elc | CV, EIS | K562 | ssDNA | AuNPs/MWCNTs | 1×102–1×107cells/mL | 60cell/mL | 112 |
| CML | Elc | CV, EIS | cDNA | ssDNA | PICA/ZnO nanocomposite | 10−15 to 10−9mol/L | 2.2×10−16mol/L | 81 |
| CML | Elc | CV | RNA from the blood samples | pDNA | Polyaniline nanotube | 10−16M | 32 | |
| CML | Elc | CV | b3a2 sequence DNA | Specific DNA capture probe | rGO nanoparticle | 1aM | 1, 10aM | 113 |
| CML, ALL | Elc | CV, EIS | DNA target | DNA probe | AuNPs PANI | 69.4aM | 65 | |
| CML, ALL | Elc | CV | CTC of HL-60, K562 | anti-MUC1 aptamers | PtAuRh Nanospheres, AuNP | 1cellmL−1 | 98 | |
| ALL | Elc | DPV, CV, EIS | Target DNA | ssDNA | nanodots, AuNP | 1.5pM, 0.26pgmL−1 | 91 | |
| ALL | Elc | DPV | CCRF-CEM | sgc8c aptamer | AuNP, Fe3O4NP | 10–1×106cellmL−1 | 114 | |
| ALL | Elc | CV, EIS | leukemic T- | pTTBA | AuNP | 0.7nM–60μM, from 70–1500μM | 0.6±0.1nM | 115 |
| ALL | Elc | PEC | CCRF-CEM | sgc8c aptamer | AgInS2 | 150-3.0×105cells/mL | 16cells/mL | 116 |
| ALL | Elc | EIS, ECL | CCRF-CEM | Aptamer | Ag2S quantum dots | 50-1×106cells/mL | 10cells/mL | 117 |
| ALL | Elc | CV, EIS | CCRF-CEM | Aptamer | CuS-graphene nanocomposite | 50-1×106CellmL−1 | 18cellmL−1 | 82 |
| ALL | Elc | DPV, EIS, CV, chronoamperometry | DNA target | catechol | AuNP | 100.0μM to 10.0pM | 1pM | 14 |
| ALL | Elc | EIS | HL 60 | Aptamer KH1C12 | AuNP | 2.5×101 to 5×105cellsmL−1 | 250cells/mL | 118 |
| ALL | Elc | CV, EIS | CCRF-CEM | PTCA/aptamer | MWCNTs, Pdnano | 10-5.0×105cells/mL | 8cells/mL | 79 |
| ALL | Elc | PEC | CCRF-CEM | Sgc8c aptamer | ZnO nanodisks @g-C3N4QDs | 20 to 20,000cell/mL | 20cell/mL | 33 |
| ALL | Elc | DPV, CV, EIS | HL 60 | KH1C12 | AuNP | 500-7.5×107cells/mL | 4cells/10mL | 119 |
| ALL | Elc | SWV | CCRF-CEM | sgc8c aptamer | AuNP | 5 to 500cells/mL | 3, 4cells/mL | 42 |
| ALL | Elc | CV | Molt-4 | Lectin liposome | Graphene NPs | 5000cells⋅mL−1 | 120 | |
| APL | Elc | CV, EIS, DPV | DNA target | DNA probe | Carbon Dots/GO Nanocomposites | 2.50×10−10M –2.25×10−9M | 83pM | 83 |
| Basophilic leukemia | Elc | CV | Histamine from RBL-2H3 | MmDH | carbon nanofibers (CSCNFs) | 0.1μM | 99 | |
| Lymphoma cancer | Elc, Opt | CV, EIS, Flrsc | HL-60, KCL-22 | anti-CD20 antibodies | Magnetic graphene nanoribbons | 100–106cells/mL | 38cells/mL | 100 |
| Not specific | Elc, Opt | CV, EIS, ECL | mRNA (miR-16) | DNA probe | AuNP | 1×10−16–1×10−7mol/L | 4.3×10−17mol/L | 35 |
| Not specific | Elc | PEC | tDNA | hairpin like DNA (HP1) | Ag@AgX Nanowires | 0.0454μM/nA | 0.033pM | 101 |
3. Future Developments, Challenges
3.1. Selectivity, sensitivity, detection techniques, and biocompatibility of leukemia biosensor
The selectivity of a biosensor is highly dependent on the receptor used. The receptor will bind to the detection target specifically. The sensor is said to be good enough for detection if it has high selectivity towards certain biomolecules. Leukemia nanobiosensor can be developed for early detection with high selectivity of specific leukemia by utilizing the bond between samples in the form of nucleic acid, leukemic cells, and other leukemia biomarkers and their bioreceptors. Based on the cell and nucleic acid as biomaker and its aptamer, the classification of types of leukemia is in Table 2.
| Type of leukemia | Type of biomarker | Biomarker | Bioreceptor | Reference |
|---|---|---|---|---|
| ALL | Cells | CCRF-CEM Molt-4 | sgc8c anti-CD5 antibodies | 50, 118 |
| Nucleic Acid | 5′-GAA GGG CTT TTG AAC TCT-3′ | 5′-Thiol-TTT TTT AGA GTT CAA AAG CCC TTC-3′ | 14 | |
| AML | Cells | HL60 KG1a | KH1C12 CD123 antibody | 27, 86 |
| Nucleic Acid | FLT3 gene | DNA probe | 34 | |
| CLL | Cells | Sel jurkat | sgc8c | 10 |
| Nucleic Acid | ZAP70 gene | ZAP70 probe | 109 | |
| CML | Cells | MS-5 cells K562 | NOX-A12 AVR2 or monoclonal anti-P-glycoprotein antibodies | 10, 37, 76 |
| Nucleic Acid | BCR/ABL fusion genes and b3a2 sequence DNA | DNA probe | 41, 65, 113 |
Based on the Table 1, Leukemia detection using optical and electrochemical techniques has been more developed compared to other techniques. The electrochemical method with EIS and CV testing is the most widely used leukemia detection method. This can be influenced because the detection technique developed can be carried out simply, but for further development of non-invasive testing using this method, it needs to be reconsidered because the patient’s DNA and blood cells are needed for accurate and specific detection. Gold nanoparticles are very widely used for the preparation of biosensors because they have a high surface area and are easy to conjugate with biomolecules through Au-S bond or have high biocontability.70 However, other materials that can be used as blood cancer biosensors are silver, semiconductors, silicon, carbon, graphene, and other metals.27,73,104,105 Metallic materials are widely used for the preparation of biosensors because the high conductivity of the material can increase the electrodes for channeling electrons in electrochemical testing.121 In addition, semiconducting and non-metallic materials, especially in nano mode, can also be used for blood cancer biosensors because of their large surface area, high extinction coefficient and fast electrical carrier mobility.30 By comparing the results of the literature study, with proper surface modification, non-metallic nanoparticles can have a higher sensitivity when compared to AuNPs.76,94 The combination of various types of nanomaterials into one blood cancer biosensor can also increase its sensitivity because it has good performance in cell affinity transduction due to the larger effective surface area.112
3.2. Sensitivity enhancement via nanomaterials developments
Nanomaterials as the main element in electrochemical and optical-based biosensors is important to be developed to obtain high sensitivity sensor. As we explained in previous section, many types of nanomaterials could be implemented in electrochemical and optical-based biosensors. Shape, morphology are the most important point since they are related to their surface property that could determine the sensitivity. The optoelectronics properties of the nanoparticles also could be optimized via modification of shapes, morphology because the optoelectrical properties of nanomaterials is strongly related to collective oscillation of electrons.122 For instance, previous report shows that the Ag nanorods with different aspect ratio, length could have different sensing ability because of its different electromagnetic dipole.123 Previous report also indicated that while the Au nanorods, Au nanocages, hollow Au nanospheres only exhibit near-infrared absorption, the AuNPs instead, could absorb the visible SPR. Thus, the AuNP shows better abilities for its applications in electrochemical and optical-based biosensors.124 The shapes, morphology of some nanomaterials could tune the fluorescence emission which important for the application of nanomaterials for electrochemical and optical-based biosensors.74
Nanocomposites approach also could be used to enhance the sensitivity of electrochemical and optical-based biosensors. Nanocomposite approach also could add some aspect that could not be obtained in some nanomaterials; therefore, this approach can perfect the sensing ability.125,126 For instance, graphene which is widely used in electrochemical biosensor has relatively poor liner range, sensitivity, thus, strategy with mixing graphene with some metal nanoparticles could usually induce higher surface area of electrochemical activity, generate electron transfer rate to enhance the sensitivity of current response.127 The nanocomposite approach also success to enhance the sensitivity of polymer bases electrochemical sensor. Polymer based electrochemical sensor usually was combined with metal nanoparticle to enhance electrical conductivity, improve the surface area, thus eventually enhance the sensitivity.128
Moreover, in most case, nanomaterials for biosensors are further transformed into a layer deposited into some substrate to form a sensor device. In the layer form or thin film, the nanomaterials further can be developed, including their shapes, morphology, material composition, thickness, even crystal structures of the nanomaterials to have better properties, mostly sensing ability. It was well recognized that the thickness, morphology or roughness of the nanomaterials for sensor in the layers or thin film form is strongly related to the amount of adsorbed protein, which mean the sensing abilities of the detected substances.129,130 Another report also shows that crystal defect on the layer surface of the sensor could play important role in the detection ability via electrochemical mechanism. Many developments of the biosensor using nanotechnology (including nanomaterial in the form of nano particle or thin film) could be initiated further linearly with increasing demand of highly sensing electrochemical and optical-based biosensors.131,132
3.3. Fabrication of biosensor using nanotechnology
Nanomaterials could be synthesized by many methods, both top-down or bottom-up techniques. Mostly, nanomaterial fabrication is conducted by chemical synthesize approach or wet-chemical technique. By using this method, we can obtain easily various types of nanomaterials; composition, phase, morphology, defect, etc. In this approach, the nanomaterial is formed via chemical solvent that can act as a stabilizer, reduction, capping agents. Nevertheless, chemical synthesis methods are mostly toxic, low-yields compared to the physical method, let say ball milling method that can be used to fabricate high-yield of nanomaterials, industrial-suited. In this method, the nanoparticle is formed via mechanical force applied during synthesis process. Another method beside chemical, physical synthesis approach is biological synthesis method. This method is more environmentally benign, non-toxic, low-cost. The abovementioned synthesis methods are used to obtain nanoparticle precursor, which further transformed into biosensor devices. The synthesis method indeed is important to obtain the nanomaterial having excellent properties, especially sensing ability. Proper choice of nanomaterial synthesis could determine what kind of nanomaterial’s phase, shape, morphology, etc we want to obtain which then affect the desire properties of the nanomaterials we required for biosensor. Nonetheless, proper choice of synthesis method also important for real application of nanomaterial itself for biosensor, let say the price. The implementation of nanomaterials for biosensor is also lack of their high-price due to expensive synthesis methods that mostly required expensive tools, substances. Proper choice of fabrication methods also related to the environmental issue, therefore more environmentally-friendly synthesis methods is also very important.
Moreover, proper choice of thin film deposition methods also significant, in the step where the nanomaterials is formed into a device. Similar to that precursor nanomaterial synthesis, thin film deposition also could be conducted through chemical, physical method, while the biological-based deposition method is very rare, even very difficult. Chemical-based thin film deposition methods include chemical vapor deposition (CVD), atomic layer deposition (ALD), Langmuir Blodgett, etc as shown in the Figs. 6(a)–6(c).133,134,135 While the physical deposition methods included pulsed laser deposition (PLD), sputtering, molecular beam epitaxy (MBE), etc as shown in the Figs. 6(d)–6(e).136,137,138 There are also the so-called vacuum, non-vacuum deposition methods. Those abovementioned deposition methods are mostly vacuum deposition methods because the deposition process is conducted in the vacuum-condition. While, others, such as namely spray coating, inkjet printing, spin coating, etc. are included in non-vacuum deposition methods. Again, proper choice of deposition method is important, not only related to obtain the desire thin-film required to obtain high-sensitivity biosensor, but also related to how the biosensor device, later, could close to the realization, commercialization. For instance, the non-vacuum deposition methods are mostly cheaper compared to the vacuum deposition methods. Non-vacuum deposition methods also can be developed easily for industrial-suited fabrication. However, if considering the purity of the thin-film, the vacuum-deposition methods are more suitable. To obtain nanostructures like those in Figs. 5(c), 5(f), 5(g), 5(j), and 5(i), an additional process is needed before deposition on the substrate, namely a printing process using a certain template. The process of making nanostructures can be done by attaching a template to a substrate and then carrying out the deposition and release process of the template to form a nanostructure. This process can be seen in Fig. 6(g).139
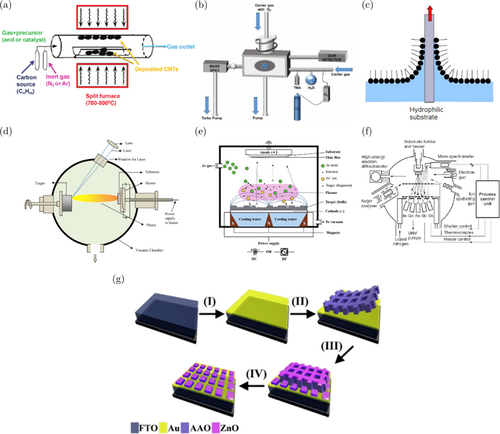
Fig. 6. Deposition method for fabrication of nanotechnology (a) chemical vapor deposition (CVD); figure adopted from Ref. 133 with permission; copyright @ 2016 Intech, (b) atomic layer deposition (ALD); figure adopted from Ref. 134 with permission; copyright @ 2018 Advanced Materials Interfaces, (c) Langmuir Blodgett; figure adopted from Ref. 135 with permission; copyright @ 2010 materials, (d) pulsed laser deposition (PLD); figure adopted from Ref. 136 with permission; copyright @ 2012 Materials Science Forum, (e) sputtering; figure adopted from Ref. 137 with permission; copyright @ 2020 Intech, (f) molecular beam epitaxy (MBE); figure adopted from Ref. 138 with permission; copyright @ 2007 University of Tartu, and (g) and fabrication of nanostructures with templates; figure adopted from Ref.139 with permission; copyright @ 2023 Molecules.
3.4. Biosensors design
To date, non-invasive biosensor lack-able causes forced by sensitivity issues. Moreover, currently developing biosensors are not portable enough to be used as a real-time detection tool for optimal disease monitoring. Research conducted by Chen et al. in 2017 made skin-like biosensor electrochemical utilizes electrodes for diagnose of blood diseases, namely diabetes.140 The same method can also fabricate for other blood diseases like leukemia. By used battery paper, very thin nanostructures can be attached to the skin adjacent to the pulse, equipped with a bioreceptor to increase the absorption of biomarkers shown in Fig. 7(a). Where the counter electrodes used are Au, Pt. While the working electrodes used are nanomaterials, bio-receptors as found in Fig. 7(b).
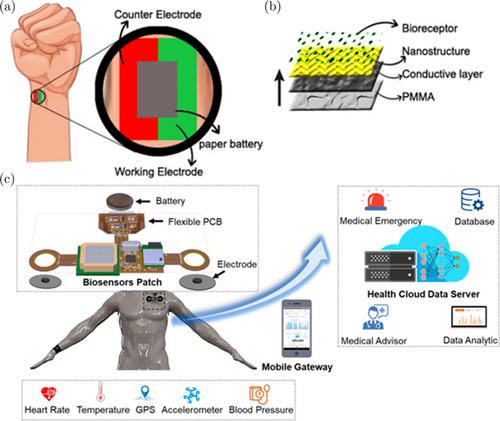
Fig. 7. (a) Typical utilization paper-like biosensor detection leukemic cells on pulsation, (b) Illustration of configuration working electrode designed to be placed on the outside of the skin, (c) IoT-based biosensor design, figure adopted from Ref. 142 with permission.
Making paper-like leukemia biosensors can be used for the development of non-invasive leukemia biosensors that can monitor the number of leukemic cells in the bloodstream. This device be obtained by utilizing biotargets in the form of blood cells that flow through the inner surface of the skin, bio-receptors according to the type of leukemia you are suffering from. The reading from this system is in the form of an electric current that can be substitute into the concentration of leukemic cells that bind to the bioreceptor. This device is worthwhile for monitoring the condition of leukemia patients who require continuous monitoring anytime, anywhere to find out the health developments of leukemia patients. This type of biosensor can be connected to the IoT system so that it can be read continuously by the user. This IoT-integrated biosensor system has been widely developed.141 The design of a biosensor for monitoring the condition of leukemia sufferers can be built in accordance with the research of Phan et al. in 2022 with a design like Fig. 7(c).142
Currently, there have been several studies related to biosensors with IoT integrated systems for detecting various diseases by placing biosensors in various body organs, for example skin, eyes, tongue, etc.143 Placing an electrochemical biosensor tattoo on the skin can be used to detect blood glucose concentrations from the sweat and the results can be read on the user’s smartphone.144 Apart from that, research conducted by Lin, Y.-R. et al. in 2018 utilized tears by making a glucose biosensor in the form of a contact lens. The more glucose that sticks to the contact lens bioreceptors, the greater the thickness of the lens and the thickness will be measured via the cellphone camera so that the user’s glucose concentration can be determined.145 Apart from that, there is also a smart sensing system by implanting biosensor chips in the teeth and tongue with an IoT integrated system for detecting glucose levels, alcohol levels, salt, sugar, pH, and temperature in saliva with electrochemical, optical, and radio frequency sensor systems.146,147,148
3.5. Lowering the toxicity, enhance the biocompatibility of nanomaterials
Several important factors should be developed for the implementation of nanomaterials in cancer detection besides the sensitivity, such as decreasing the toxicity, increase the biocompatibility of the nanomaterials. These two factors are important since the sensor device is used directly into the body, one may be integrated with the body (see Fig. 6). Proper choice of the non-toxic nanomaterials is important, for instance, instead of use CdTe, we can use Au as the component in the biosensor, since Au has lower toxicity compared to CdTe.149 Furthermore, though Au has low toxicity, however, if we synthesize it with highly toxic chemical routes, it can induce the toxicity in the Au originated from the small amount toxic chemical residue that my still attached in the Au during the synthesis process. Therefore, proper choice of nanomaterials synthesis is also pivotal importance.150 Recently, the so-called green synthesis approach appears as the shortcut to obtain a nontoxic nanomaterial.151 In this case, the nanomaterials are fabricated using the non-toxic solvent or biological agents that could act as a reduction, capping, stabilization agents to obtain a nanomaterial. Green synthesis also environmentally benign, low-cost, simple process. A strategy to decrease toxicity, enhance the biocompatibility of nanomaterials also could be carried out by structural modification. It is known previously that the shapes, morphology of nanomaterials also affect their toxicity which strongly related to nanomaterial’s surface property.152 Proper choice of nanomaterials shape, morphology is important to increase the feasibility of nanomaterials for biosensors, which not only related due to the properties of some nanomaterials that required for biosensors performance, such as sensitivity, but also related to the toxicity, biocompatibility of some nanomaterials which important for their direct utilization with the human body when they integrated as biosensors.
Acknowledgments
The authors are grateful for the financial support from the Institut Teknologi Sepuluh Nopember under the project scheme of BRIN awards number: 6/IV/KS/05/2023.
Conflicts of Interest
The authors declare that there are no conflicts of interest relevant to this article.
ORCID
Tahta Amrillah  https://orcid.org/0000-0002-5333-7980
https://orcid.org/0000-0002-5333-7980
Nasori Nasori  https://orcid.org/0000-0002-9293-530X
https://orcid.org/0000-0002-9293-530X