Evaluation of Pregnancy Outcomes of Vitrified-Warmed Blastocyst Transfer before and after Endometrial Receptivity Analysis in Identical Patients with Recurrent Implantation Failure
Abstract
Background: The clinical value of personalized embryo transfer (pET) guided by the endometrial receptivity analysis (ERA) tests for recurrent implantation failure (RIF) cases is still unclear. The aim of this study is to clarify the efficacy of ERA leading to personalization of the day of embryo transfer (ET) in RIF patients.
Methods: A retrospective study was performed for 94 patients with RIF who underwent ERA between July 2015 and December 2019. Pregnancy outcomes in a previous vitrified-warmed blastocyst transfer (previous VBT) and a personalized vitrified-warmed blastocyst transfer (pVBT) in identical patients were compared. The details of each pVBT were further analyzed between patients in a non-displaced group, which indicated “receptive” cases in ERA results and those who were in the displaced group, which indicated “non-receptive” cases.
Results: When the pregnancy rate, both per patient and per transfer cycle, of previous VBT and pVBT were compared, a significant increase in pVBT was observed between the two methods (5.3% vs. 62.8%, 4.4% vs. 47.9%, respectively). The pregnancy rates, implantation rates, and clinical pregnancy rates of the first pVBT were significantly higher in the displaced group than the non-displaced group. The cumulative ongoing pregnancy rate of the displaced group tended to be higher compared to that of the non-displaced group in the first pVBT, although the difference was not statistically significant (51.0% vs. 31.1%, ).
Conclusions: Our study demonstrates that pVBT guided by ERA tests may improve pregnancy outcomes in RIF patients whose window of implantation (WOI) is displaced, and its effect may be more pronounced at the first pVBT. The displacement of WOI may be considered to be one of the causes of RIF, and its adjustment may contribute to the improvement of pregnancy outcomes in RIF patients.
INTRODUCTION
Recurrent implantation failure (RIF) is an unaddressed major cause of infertility in otherwise healthy women and one that has remained poorly characterized (Margalioth et al., 2006); Simon et al., 2012). Various definitions of RIF exist, but one expert proposed pathologic implantation failure be defined as failure of three IVF cycles in which one or two high-grade quality embryos were transferred to the patient in each cycle (Simon et al., 2012) or after two failures in oocyte donor recipients. There are several causes of RIF, such as pathologic alterations of the endometrial cavity (e.g., hyperplasia, submucosal myomas, endometrial polyps, endometritis, synechia), hydrosalpinx, embryonic aneuploidy, thrombophilias, and systemic factors, such as thyroid dysfunction (Ruiz-Alonso et al., 2013). Recently, other causes of RIF have been reported, such as immunological factors, altered molecules, infection, including chronic endometritis (CE), and endometrial receptivity (Bashiri et al., 2018). Although embryonic aneuploidy is likely to be the major contributor to human implantation failure, especially in cases of advanced maternal age (Kung et al., 2015), it has been reported that the proportion of euploid embryos failing to implant has been 50%–60% (Harton et al., 2013), which might suggest the importance of the endometrium and its receptivity status as another dominant factor for implantation failure (Fox et al., 2016).
Endometrial receptivity is characterized by a finite and time-sensitive window of implantation (WOI) orchestrated by an incompletely defined complex of endocrine, paracrine, and autocrine factors (Cha et al., 2012). Recently, the endometrial receptivity analysis (ERA) using the transcriptomic signature of endometrial receptivity composed of 248 genes has been applied clinically. The ERA was developed as a means of personalizing embryo transfer (pET) timing, particularly in cases of RIF where endometrial receptivity may play a dominant factor. The accuracy of the ERA test is superior to endometrial histology, and results were reproducible in the same patients 29–40 months after the first test (Diaz-Gimeno et al., 2013). Simon et al. reported in a large, prospective, multicenter randomized controlled trial (RCT) results indicating the benefit of pET compared to frozen embryo transfer (ET) and fresh ET at the first appointment (Simon et al., 2020). In this RCT, cumulative live birth rates after 12 months were significantly higher in the pET group (71.2%) compared with frozen ET (55.4%) and fresh ET (48.9%) groups. Although this RCT strengthened the clinical utility of pET guided by ERA, RIF cases were not included in this RCT. Therefore, there is still lack of evidence for the clinical application of ERA to RIF cases.
The aim of this study was to clarify the efficacy of ERA leading to personalization of the day of ET in RIF patients.
METHODS
We conducted a two-center retrospective study, in a private fertility clinic, including patients who agreed to undergo an ERA from July 2015 to December 2019. The decision to perform the ERA followed a discussion between the patient and her physician. This study was approved by the Institutional Review Board of Kyono ART Clinic, Sendai and Takanawa, Japan (submission number: #4502-200501). All the patients who were involved in this study allowed the researchers to use their medical record data for research in an unidentifiable manner. Written, informed consent was obtained from all the patients prior to the ERA in the two centers.
Patients and samples
RIF patients (who had histories of three or more ET failures) with at least one vitrified-warmed blastocyst transfer (VBT) were included in this study. All the patients were routinely examined by vaginal ultrasound (hysteroscopy if necessary), for thyroid function, thrombophilia (protein S, protein C, antithrombin III, coagulation factor XII, lupus anticoagulant, anticardiolipin antibodies), and were treated appropriately if any disorder was found.
The cases aged over 38 years old were excluded. Also, confounding factors such as multipara, nonoperated hydrosalpinx, congenital uterine anomaly, untreated submucous myomas or endometrial polyps, endometrial hyperplasia, severe male factor infertility ( million spermatozoa/mL), and abnormal karyotypes of both partners were excluded. Cases with the second ERA, spontaneous pregnancy after ERA, and those who underwent only cleavage stage ETs, only natural cycle blastocyst transfers, or only fresh blastocyst transfers after ERA were also excluded from this study (Fig. 1). Embryos were vitrified at the blastocyst stage with a diameter of 160–170m and after assisted hatching one or two embryos more than 5BC were transferred considering the time from transfer to invasion. The quality was assessed by an experienced embryologist with the use of the grading system proposed by Gardner (1999).
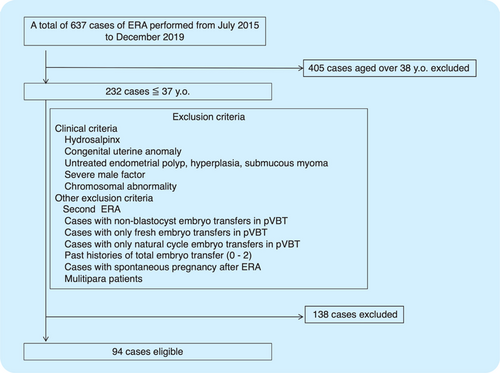
Fig. 1. Patient flowchart.
Notes: A total of 637 patients underwent ERA in our centers during the study period. According to the exclusion criteria listed below, 94 patients were eligible for the study.
Endometrial sampling and processing
The ERA was performed in a hormonal replacement therapy (HRT) cycle. In an HRT cycle, after appropriate priming with estradiol (by transdermal patch, estradiol valerate, or both when necessary) for 1–2 weeks, leading to a trilaminar endometrium of mm and confirmation of the appropriate hormonal status, P was administered (either by micronized suppositories at 300mg per day or by chlormadinone acetate at 12mg per day) for five full days, and at P+120h (P+5), an endometrial biopsy was performed. The same P was administered at the time of ERA and personalized vitrified-warmed blastocyst transfer (pVBT) in all patients. If the serum progesterone levels were ng/mL at the time of P initiation, the relevant cycle was cancelled. The endometrial biopsy was performed from the uterine fundus using a catheter called ENDOSUCTION (Hakko Company, Ltd., Nagano, Japan) at P+120h (P+5) in the HRT cycles as described earlier. The biopsied endometrial sample was put into a cryotube containing 1.5mL RNA later (Quiagen, Tokyo, Japan) and then shaken for a few seconds. It was kept at 4∘C for 4h and shipped at room temperature for the ERA analysis (Igenomix, Valencia, Spain/Igenomix Japan, Tokyo, Japan) (Hashimoto et al., 2017).
WOI recommendation according to the endometrial receptivity array prediction
The ERA demonstrates each patient’s individual WOI as “receptive” (optimal in P+5), “early receptive” (optimal 12h later from P+5), “late receptive” (optimal 12h earlier from P+5), or “nonreceptive,” which is further classified into “pre-receptive” (optimal 24h or more later from P+5) or “post-receptive” (optimal 24h earlier from P+5), respectively. Accordingly, in a subsequent cycle, we performed VBT at P+6, P+5.5, P+5, P+4.5, or P+4 days in the patients who were at the pre-receptive, early receptive, receptive, late receptive, or post-receptive stage, respectively. In cases with “post-receptive” or “pre-receptive 2 days” results, re-biopsy was suggested from Igenomix.
In this study, the cases with ERA results of “receptive” were defined as the “non-displaced” group, and all other cases were defined as “displaced” group. Vitrified blastocysts were warmed and transferred according to ERA results. Patients with a receptive endometrium underwent VBT in an HRT cycle mimicking the ERA cycle. In patients with a modified implantation window, VBT was adjusted in subsequent cycles based on the personalized WOI identified by ERA (pVBT). VBT performed before ERA was defined as previous VBT.
Pregnancy outcome measures
The pregnancy rates (PR) of previous VBT and pVBT were compared. Pregnancy outcomes of pVBT were also compared between patients in the non-displaced group and the displaced group. Clinical pregnancy was defined as the confirmation of a gestational sac in the uterine cavity by ultrasound analysis. Implantation rate (IR) was the number of gestational sacs observed by vaginal ultrasound at the fifth week of gestation. Ongoing pregnancy rate (OPR) was defined as each pregnancy showing a positive heartbeat at ultrasound after 12 weeks of gestation. Clinical miscarriage rate was the number of spontaneous pregnancy losses in which one or more gestational sacs were previously observed. Ectopic PR was the number of pregnancies outside the uterine cavity, diagnosed by ultrasound, surgical visualization, or histopathology. Cumulative ongoing pregnancy rate (COPR) was the number of patients with ongoing pregnancy. The pregnancy outcomes of all recruited patients were followed up until June 2020.
Statistical analysis
The statistical analysis was performed using the paired t test, Mann-Whitney U test, or Fisher’s exact test where appropriate. Comparing PR between previous VBT and pVBT, the data were transformed using the arcsine square root to obtain a normal distribution before analysis. A p-value of was considered to be statistically significant.
RESULTS
Patient characteristics
A total of 637 cases underwent ERA in our centers during the study period, 405 patients over the age of 38 were excluded, and 138 cases were ineligible according to the other exclusion criteria. Finally, a total of 94 patients with RIF were included in this study (Fig. 1).
As shown in Table 1, the average age of the 94 patients with RIF was (28–37) years; the past histories of total failed ETs and VBTs were cycles and cycles, respectively. The ERA biopsy result showed a receptive profile (non-displaced WOI) in 47.8% (45/94) and displaced WOI in 52.1% (49/94). Of the displaced group results, 33.0% (31/94), 14.9% (14/94), 2.1% (2/94), and 2.1% (2/94) indicated a pre-receptive, early receptive, late receptive and post-receptive states, respectively.
| Patients | 94 |
| Age (years±SD) | 34.5±2.2 |
| BMI (kg/m2) | 20.9±2.7 |
| Gravida | 0 (0–3) |
| The average past total ET before ERA | 5.3±2.3 |
| The average past VBT before ERA | 4.0±2.1 |
| ERA results | |
| Non-displaced | 45 (47.8) |
| Displaced | 49 (52.1) |
| Pre-receptive (P6) | 31 (33.0) |
| Early receptive (P5.5) | 14 (14.9) |
| Late receptive (P4.5) | 2 (2.1) |
| Post-receptive (P4) | 2 (2.1) |
| Days from biopsy to the first pVBT (median, Q1–Q3) | 86.5 (52–128) |
Comparing the PR of previous VBT vs. pVBT
When the PR, both per patient and per transfer cycle, of previous VBT and pVBT were compared, a significant increase in pVBT was observed. The PR of pVBT per patient significantly increased compared to previous VBT (62.8% vs. 5.3%, ) ( Table 2). When the PR per patient before and after ERA was further analyzed between the non-displaced group and the displaced group, there were statistically significant differences in both groups (4.9% vs. 55.9%, , 5.6% vs. 69.1%, , respectively). Similarly, among the PR per transfer cycle of previous VBT and pVBT, pVBT had a markedly higher PR (previous VBT, 4.4% vs. pVBT, 47.9%, ). In both the non-displaced and the displaced groups, PR per transfer cycle before and after ERA was significantly increased (previous VBT, 2.7% vs. pVBT, 44.4%, , previous VBT 6.0% vs. pVBT 51.9%, , respectively).
| Previous VBT | pVBT | pvalue | |
|---|---|---|---|
| Number of cycles | 364 | 169 | — |
| Non-displaced | 182 | 90 | — |
| Displaced | 182 | 79 | — |
| Average number of VBT | |||
| Non-displaced | 4.33±2.39 | 2.00±0.95 | |
| Displaced | 3.87±1.90 | 1.61±1.15 | |
| Pregnancy rate per patient (%) | |||
| Non-displaced | 4.9 | 55.9 | |
| Displaced | 5.6 | 69.1 | |
| Pregnancy rate per transfer (%) | |||
| Non-displaced | 5 (2.7) | 40 (44.4) | |
| Displaced | 11 (6.0) | 41 (51.9) | |
Comparing the pregnancy outcome of pVBT between non-displaced and displaced groups
To demonstrate the efficacy of pVBT guided by ERA, further subgroup analysis in each pVBT was performed. As shown in Table 3, there were no differences in the backgrounds of the non-displaced group and the displaced group in terms of age, BMI, gravida, or the number of past VBT before ERA. PR (44.4% vs. 51.9%, ), IR (42.9% vs. 51.9%, ), CPR (43.3% vs. 50.6%, ), and OPR (34.4% vs. 38.0%, ) in all pVBTs were higher in the displaced group, although this difference was not statistically significant ( Table 3). To further investigate the individual effect of WOI displacement between the VBT cycles, we performed a subgroup analysis among the first to the third or more pVBTs.
| Non-displaced | Displaced | pvalue | |
|---|---|---|---|
| Patients | 45 | 49 | — |
| Age (years±SD) | 34.7±1.8 | 34.2±2.6 | 0.63a |
| BMI (kg/m2) | 20.6±2.2 | 21.2±3.1 | 0.36a |
| Gravida | 0.51±0.73 | 0.45±0.74 | 0.89a |
| All pVBT | |||
| Number of cycles | 90 | 79 | — |
| Pregnancy rate | 40 (44.4) | 41 (51.9) | 0.36b |
| Implantation rate | 39 (42.9) | 42 (51.9) | 0.28b |
| Clinical pregnancy rate | 39 (43.3) | 40 (50.6) | 0.36b |
| Ongoing pregnancy rate | 31 (34.4) | 30 (38.0) | 0.75b |
| Clinical miscarriage rate | 8 (20.0) | 10 (24.4) | 0.80b |
| Ectopic pregnancy rate | 1 (2.5) | 1 (2.4) | 1.00b |
| First pVBT | |||
| Number of cycles | 45 | 49 | — |
| Pregnancy rate | 18 (40.0) | 33 (67.3) | ¡0.05b |
| Implantation rate | 18 (39.1) | 34 (68.0) | ¡0.05b |
| Clinical pregnancy rate | 18 (40.0) | 32 (65.3) | ¡0.05b |
| Ongoing pregnancy rate | 14 (31.1) | 25 (51.0) | 0.06b |
| Clinical miscarriage rate | 8 (20.0) | 7 (21.2) | 1.00b |
| Ectopic pregnancy rate | 1 (2.5) | 1 (3.0) | 1.00b |
| Days from biopsy to the first pVBT | 84 (51–130) | 89 (53.5–127) | 0.76a |
| Second pVBT | |||
| Number of cycles | 27 | 14 | — |
| Pregnancy rate | 10 (37.0) | 3 (21.4) | 0.48b |
| Implantation rate | 11 (36.7) | 3 (21.4) | 0.49b |
| Clinical pregnancy rate | 10 (37.0) | 3 (21.4) | 0.48b |
| Ongoing pregnancy rate | 9 (33.3) | 2 (14.3) | 0.28b |
| Clinical miscarriage rate | 1 (10.0) | 1 (33.3) | 0.42b |
| Ectopic pregnancy rate | 0 (0) | 0 (0) | 1.00b |
| Days from biopsy to the second pVBT (median, Q1–Q3) | 197 (120–230.5) | 193.5 (138–315) | 0.51b |
| Third or more pVBT | |||
| Number of cycles | 18 | 16 | — |
| Pregnancy rate | 12 (66.7) | 5 (31.3) | 0.08b |
| Implantation rate | 10 (58.8) | 5 (29.4) | 0.17b |
| Clinical pregnancy rate | 11 (61.1) | 5 (31.3) | 0.10b |
| Ongoing pregnancy rate | 8 (44.4) | 3 (18.8) | 0.15b |
| Clinical miscarriage rate | 3 (25.0) | 2 (40.0) | 0.60b |
| Ectopic pregnancy rate | 1 (8.3) | 0 (0) | 1.00b |
| Days from biopsy to third or more pVBT (median, Q1–Q3) | 341.5 (263–572) | 389.5 (322.5–557) | 0.40a |
PR (40.0% vs. 67.3%, ), IR (39.1% vs. 68.0%, ), and CPR (40.0% vs. 65.3%, ) of the first pVBT were significantly higher in the displaced group than the non-displaced group ( Table 3). OPR (31.1% vs. 51.0%, ) was higher in the displaced group than the non-displaced group, although this difference was not statistically significant. Conversely, PR, IR, CPR, and OPR of the second pVBT and the third or more pVBT in the displaced group were lower than in the non-displaced group, although this difference was not statistically significant (37.0% vs. 21.4%, , 36.7% vs. 21.4%, , 37.0% vs. 21.4%, , 33.3% vs. 14.3%, , respectively) ( Table 3). Clinical miscarriage rate, ectopic PR, and days from biopsy to the each pVBT were similar between the non-displaced group and the displaced group. COPR of the displaced group tended to be higher compared to the non-displaced group in the first pVBT (51.0% vs. 31.1%, ), although the difference was not statistically significant (Fig. 2).
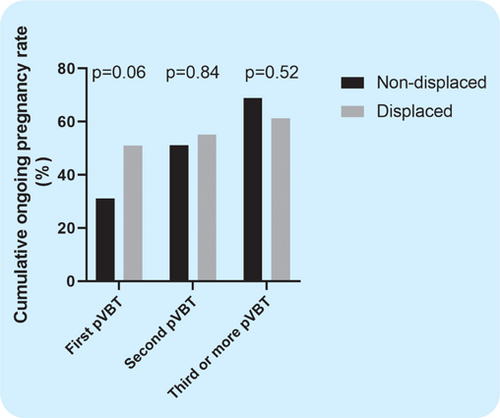
Fig. 2. Cumulative ongoing pregnancy rate in pVBT.
Notes: COPR was the number of patients with ongoing pregnancy divided by the total number of patients in the non-displaced group or displaced group. The black bar indicates COPR in the non-displaced group and the gray bar indicates COPR in the displaced group. p ¡ 0.05 is significant.
DISCUSSION
To the best of the authors’ knowledge, there has been no other study to report on the efficacy of the ERA and pVBT guided by the ERA results for patients with RIF by comparing pregnancy outcomes before and after ERA.
Recently, Simon et al. reported a large, prospective RCT results demonstrating the clinical utility of pET compared to frozen ET and fresh ET (Simon et al., 2020). In this RCT, 458 patients aged 37 years or younger undergoing IVF with blastocyst transfer at first appointment were randomized to pET guided by ERA, frozen ET, or fresh ET. The PR, IRs, and cumulative live birth rates of those patients who underwent pET guided by ERA were significantly improved compared to those patients with frozen ET or fresh ET (Simon et al., 2020). However, patients with RIF were not included in this RCT. Although previous studies have reported the clinical impact of the ERA test both in RIF and non-RIF populations yielding conflicting results (Ruiz-Alonso et al., 2013; Hashimoto et al., 2017; Mahajan, 2015; Bassil et al., 2018), in our present study of RIF cases, a remarkable improvement in VBT’s PR was observed after adjusting the WOI according to the result of ERA ( Table 2), indicating that WOI displacement may be one of the important factors of RIF. There may be some reasons why the PR of pVBT was markedly improved. The pVBT was performed at the designated time according to ERA results, but previous VBT was not precisely controlled because previous VBTs included natural cycle VBTs and cases performed in other institutions with different operations. In our study, ERA tests were performed by HRT at P+120h in all cases in order to conduct pVBT more precisely.
Of the 61 patients in which ongoing pregnancy was finally achieved, 50 patients (82.0%) were pregnant by the second pVBT ( Table 3). Moreover, in the non-displaced group, 23 patients (74.2%) out of the 31 patients with whom ongoing pregnancy was achieved were pregnant by the second pVBT, and in the displaced group, 27 patients (90.0%) of the 30 patients were pregnant up to the second pVBT. It was considered that pVBT based on ERA results was more effective in the first or second VBT after ERA, and this tendency was stronger in the displaced group ( Table 3). This indicates that in the RIF cases with displaced WOI, the adjustment of WOI at the time of blastocyst transfer had a great impact on the achievement of pregnancy.
In this study, CPR of the first pVBT was significantly higher in the displaced group than in the non-displaced group (, Table 3); however, there was no difference in COPR between the non-displaced and displaced groups, suggesting that the effect of WOI correction may be highest in the first pVBT (Fig. 2). COPR was finally higher in the non-displaced group compared to the displaced group although the difference was not statistically significant; multidisciplinary intervention including oriental medicine may have acted effectively for the non-displaced group. On the other hand, despite the correction of WOI, about 35% of cases in our study could not achieve pregnancy (Fig. 2). Although Tan et al. analyzed the cases with whom both PGT-A and ERA was performed, OPR was 75% in non-RIF cases and 44% in RIF cases (Tan et al., 2018), suggesting that there may be other factors of implantation failure other than WOI displacement and embryonic aneuploidy. For further improvement of the pregnancy outcomes of RIF patients, appropriate interventions for other RIF factors such as immunological factors, thyroid function, and CE, and so on (Bashiri et al., 2018), as well as PGT-A should be performed in addition to the correction of WOI, especially in the non-displaced group.
Some precautions should be taken into consideration when applying the ERA test clinically. First, the stage at which the blastocyst should be transferred must be considered. Although the ERA results suggest the timing of ET every 12h, it takes about 20h from the first signs of blastulation to the start of hatching (Martínez-Granados et al., 2017). In our study, all embryos were vitrified at the blastocyst stage with a diameter of 160–170m, which means grade 4 in Gardner’s criteria, and at 4–6h after warming, they were transferred in order to match the embryonic stage and WOI more precisely. Although this is controversial (Frantz et al., 2019; Nastri et al., 2015), there are possibilities that local injury induced by an endometrial biopsy (scratching) might improve embryo implantation in the following ET cycle. Recently, an RCT reported that endometrial scratching did not increase the frequency of live birth in 1364 women who underwent IVF compared to a no-intervention group (Lensen et al., 2019). In our study, the median number of days from ERA testing to the first pVBT was more than 80 days ( Tables 1 and 3), which indicates that the endometrial injury may not have accounted for our results.
Regarding factors affecting ERA results, a recent report demonstrated that the percentage of receptive was lower in cases with CE compared to non-CE cases. It was recommended to perform the ERA test after appropriate treatment for CE (Kuroda et al., 2020). However, in their cases with CE, the number of CD138-positive plasma cells was per 10 non-overlapping random stromal areas, indicating that their cases with CE presented severe inflammation, which may have affected the ERA results. It may not be recommended to perform ERA when there is any presence of strong inflammation since the ERA test contains inflammatory-related genes (Diaz-Gimeno et al., 2011). Regarding our study, hysteroscopy is routinely performed before the initial vitrified-warmed ET in our clinic in order to macroscopically identify any uterine pathologies including CE; thus, the patients included in our study may have had appropriate interventions if any abnormalities were found. Considering that the prevalence of CE among RIF patients varies by reports due to the lack of standardized diagnostic criteria, and may be as low as 7.7%, not significantly higher than that of fertile subjects (5.0%) (Liu et al., 2018), the impact of CE upon ERA result might be limited. It might be better to diagnose CE before performing ERA, although the authors believe that further studies are necessary for analyzing the effect of CE upon ERA result.
There are several limitations in this retrospective study, including lack of a control group without ERA and small sample size as a result of excluding some confounding factors. With regard to embryonic chromosomal aneuploidy, we could not apply PGT-A in this study because PGT-A is still in clinical trials and not approved in all fertility hospitals in Japan. Therefore, we have to consider more accurate diagnosis and interventions of RIF factors other than embryonic chromosomal aneuploidy. Ideally, a larger RCT would be performed among RIF patients with and without ERA in the future.
RIF is still challenging in the field of assisted reproductive technology. We analyzed the pregnancy outcomes before and after WOI correction using the ERA test and the details of each pVBT. Our study demonstrates that pVBT guided by ERA tests may improve the pregnancy outcomes in RIF patients whose WOI is displaced, and its effect may be more pronounced at the first pVBT in those with displaced WOI. The displacement of WOI may be considered to be one of the causes of RIF, and its adjustment may contribute to the improvement of pregnancy outcomes in RIF patients.
ACKNOWLEDGMENTS
This study was not supported by any funding. The authors declare that they have no conflicts of interest. The authors thank Steve Beacall for his excellent help with English proofreading and all staff at our clinic for their assistance in the conduct of this study.
FUNDING
There is no funding for this study.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
References
- . Recurrent Implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018; 16(1) :121. Crossref, Google Scholar
- Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet. 2018; 35(7) :1301–5. Crossref, Google Scholar
- . Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012; 18(12) :1754–67. Crossref, Google Scholar
- A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011; 95(1) :50–60. Crossref, Google Scholar
- The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013; 99(2) :508–17. Crossref, Google Scholar
- . Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016; 105(4) :873–84. Crossref, Google Scholar
- Decrease in pregnancy rate after endometrial scratch in women undergoing a first or second in vitro fertilization. A multicenter randomized controlled trial. Hum Reprod. 2019; 34(1) :92–9. Crossref, Google Scholar
- .
In-vitro culture of human blastocyst . In: Jansen RMortimer D, (Eds.), Towards Reproductive Certainty: Infertility and Genetics Beyond. Carnforth: Parthenon Press; 1999 :378–88. Google Scholar - Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013; 100(6) :1695–703. Crossref, Google Scholar
- Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two-centers study. Reprod Med Biol. 2017; 16(3) :290–6. Crossref, Google Scholar
- . Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod Biomed Online. 2015; 31(6) :760–9. Crossref, Google Scholar
- Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. Immun Inflamm Dis. 2020; 8 :650–8. Crossref, Google Scholar
- A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. 2019; 380(4) :325–34. Crossref, Google Scholar
- Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018; 109 :832–39. Crossref, Google Scholar
- . Endometrial receptivity array: clinical application. J Hum Reprod Sci. 2015; 8(3) :121–9. Crossref, Google Scholar
- . Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006; 21(12) :3036–43. Crossref, Google Scholar
- Inter-laboratory agreement on embryo classification and clinical decision: conventional morphological assessment vs. time lapse. PLOS ONE. 2017; 12(8) :e0183328. Crossref, Google Scholar
- Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2015;(3) :Cd009517. Google Scholar
- The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013; 100(3) :818–24. Crossref, Google Scholar
- . Repeated implantation failure: clinical approach. Fertil Steril. 2012; 97(5) :1039–43. Crossref, Google Scholar
- A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod Biomed Online. 2020; 41(3) :402–15. Crossref, Google Scholar
- The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018; 35(4) :683–92. Crossref, Google Scholar